当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Mukaiyama Mannich Reactions of 2,5-Bis(trimethylsilyloxy)furan
Organic Letters ( IF 4.9 ) Pub Date : 2019-06-19 00:00:00 , DOI: 10.1021/acs.orglett.9b01664
Stephen W. Laws 1 , Sara Y. Howard 1 , Raquel Mato 1 , Shuyu Meng 1 , James C. Fettinger 1 , Jared T. Shaw 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-06-19 00:00:00 , DOI: 10.1021/acs.orglett.9b01664
Stephen W. Laws 1 , Sara Y. Howard 1 , Raquel Mato 1 , Shuyu Meng 1 , James C. Fettinger 1 , Jared T. Shaw 1
Affiliation
|
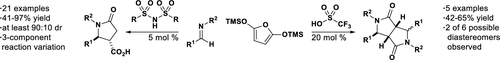
The organocatalytic synthesis of densely substituted mono- and bis-γ-lactams involving the Mukaiyama Mannich addition of 2,5-bis(trimethylsilyloxy)furan to imines is described. Use of a ditoluenesulfonylimide catalyst produces γ-lactams from monoaddition, whereas a more acidic catalyst (triflic acid) produces fused bis-lactams from double addition. Optimized organocatalytic conditions allow for the selective synthesis of either desired core as well as the one-pot, multicomponent assembly of the trisubstituted monolactams from aldehydes, amines, and bis-trimethylsilyloxyfuran. An examination of chiral acids found these organocatalysts to be highly active and diastereoselective in the monoaddition reaction, albeit with no enantioselectivity.
中文翻译:

2,5-双(三甲基甲硅烷氧基)呋喃的有机催化Mukaiyama Mannich反应
描述了稠密取代的单-和双-γ-内酰胺的有机催化合成,其中涉及向亚胺中添加2,5-双(三甲基甲硅烷氧基)呋喃的Mukaiyama Mannich。使用二甲苯磺酸磺酰亚胺酯催化剂可通过单加成反应生成γ-内酰胺,而酸性更高的催化剂(三氟甲磺酸)可通过两次添加生成熔融的双内酰胺。优化的有机催化条件允许从醛,胺和双三甲基甲硅烷基氧基呋喃选择性合成所需的核以及单取代多组分三取代单内酰胺。对手性酸的检查发现这些有机催化剂在单加成反应中具有很高的活性和非对映选择性,尽管没有对映选择性。
更新日期:2019-06-19
中文翻译:

2,5-双(三甲基甲硅烷氧基)呋喃的有机催化Mukaiyama Mannich反应
描述了稠密取代的单-和双-γ-内酰胺的有机催化合成,其中涉及向亚胺中添加2,5-双(三甲基甲硅烷氧基)呋喃的Mukaiyama Mannich。使用二甲苯磺酸磺酰亚胺酯催化剂可通过单加成反应生成γ-内酰胺,而酸性更高的催化剂(三氟甲磺酸)可通过两次添加生成熔融的双内酰胺。优化的有机催化条件允许从醛,胺和双三甲基甲硅烷基氧基呋喃选择性合成所需的核以及单取代多组分三取代单内酰胺。对手性酸的检查发现这些有机催化剂在单加成反应中具有很高的活性和非对映选择性,尽管没有对映选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号