当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rotenone Increases Isoniazid Toxicity but Does Not Cause Significant Liver Injury: Implications for the Hypothesis that Inhibition of the Mitochondrial Electron Transport Chain Is a Common Mechanism of Idiosyncratic Drug-Induced Liver Injury.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-06-19 00:00:00 , DOI: 10.1021/acs.chemrestox.9b00116 Tiffany Cho 1 , Xijin Wang 1 , Jack Uetrecht 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-06-19 00:00:00 , DOI: 10.1021/acs.chemrestox.9b00116 Tiffany Cho 1 , Xijin Wang 1 , Jack Uetrecht 1
Affiliation
|
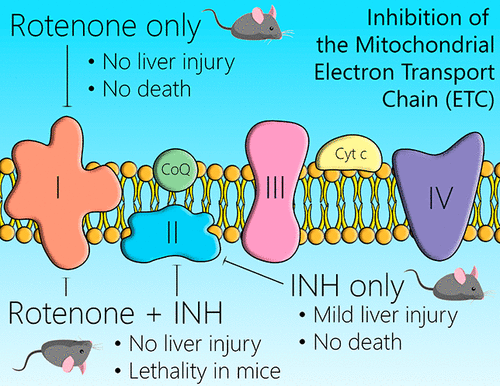
Idiosyncratic drug reactions (IDRs) significantly increase the risk of failure in drug development. The major IDR leading to drug candidate failure is idiosyncratic drug-induced liver injury (IDILI). Although most evidence suggests that IDRs are mediated by the immune system, there are other hypotheses, such as mitochondrial dysfunction. Many pharmaceutical companies routinely screen for mitochondrial toxicity in an attempt to “derisk” drug candidates. However, the basic hypothesis has never been rigorously tested. A major assay used for this screening involves measurement of inhibition of the mitochondrial electron transport chain. One study found that the combination of rotenone and isoniazid, which inhibit mitochondrial complex I and II, respectively, were synergistic in causing hepatocyte toxicity in vitro and suggested the combination of another drug that inhibited complex I would increase the risk of isoniazid-induced liver injury in patients. We tested this hypothesis in vivo where wild-type and PD-1–/– mice administered anti-CTLA-4, our impaired immune tolerance mouse model, were given 0.02% (w/v) rotenone in water or 0.1%, 0.05%, and 0.01% (w/w) rotenone alone or in combination with isoniazid in food. The cotreatment led to lethality in 100% of the animals receiving 0.1% rotenone and 0.2% isoniazid and 83% of the animals cotreated with 0.05% rotenone and 0.2% isoniazid in food. Nevertheless, there was no significant increase in GLDH or histological evidence of liver injury. No signs of toxicity were observed in any of the mice given rotenone or isoniazid alone. Even though inhibition of the mitochondrial electron transport chain did not lead to significant liver toxicity, it could provide danger signals that promote immune-mediated liver injury. However, rotenone did not significantly increase the liver injury induced by isoniazid in our impaired immune tolerance model. Overall, we conclude that inhibition of the mitochondrial electron transport chain is not a significant mechanism of IDILI.
中文翻译:

鱼藤酮增加异烟肼的毒性,但不会引起严重的肝损伤:假说线粒体电子运输链的抑制是异质性药物诱发的肝损伤的常见机制这一假说的含义。
异质性药物反应(IDR)大大增加了药物开发失败的风险。导致候选药物失败的主要IDR是特异药物性肝损伤(IDILI)。尽管大多数证据表明IDR是由免疫系统介导的,但还有其他假设,例如线粒体功能障碍。许多制药公司例行检查线粒体毒性,以试图“脱毒”候选药物。但是,从未对基本假设进行过严格的检验。用于该筛选的主要测定法涉及对线粒体电子传输链的抑制作用的测量。一项研究发现,鱼藤酮和异烟肼的组合分别抑制线粒体复合体I和II,在体外引起肝细胞毒性方面具有协同作用,并建议与另一种抑制复合物I的药物合用会增加异烟肼引起的患者肝损伤的风险。我们在体内测试了这一假设,在野生型和PD-1 – / –小鼠中,我们的免疫耐受性受损的小鼠模型抗CTLA-4被给予了水中0.02%(w / v)的鱼藤酮或0.1%,0.05% ,以及食品中异烟肼的0.01%(w / w)鱼藤酮。共同处理导致100%的动物接受0.1%鱼藤酮和0.2%异烟肼的致死性,83%的动物与食物中的0.05%鱼藤酮和0.2%异烟肼共同处理。然而,GLDH或肝损伤的组织学证据没有显着增加。在单独给予鱼藤酮或异烟肼的任何小鼠中均未观察到毒性迹象。即使抑制线粒体电子运输链不会导致明显的肝毒性,它可能会提供危险信号,促进免疫介导的肝损伤。但是,在我们受损的免疫耐受模型中,鱼藤酮并未显着增加异烟肼引起的肝损伤。总的来说,我们得出的结论是,抑制线粒体电子传输链不是IDILI的重要机制。
更新日期:2019-06-19
中文翻译:

鱼藤酮增加异烟肼的毒性,但不会引起严重的肝损伤:假说线粒体电子运输链的抑制是异质性药物诱发的肝损伤的常见机制这一假说的含义。
异质性药物反应(IDR)大大增加了药物开发失败的风险。导致候选药物失败的主要IDR是特异药物性肝损伤(IDILI)。尽管大多数证据表明IDR是由免疫系统介导的,但还有其他假设,例如线粒体功能障碍。许多制药公司例行检查线粒体毒性,以试图“脱毒”候选药物。但是,从未对基本假设进行过严格的检验。用于该筛选的主要测定法涉及对线粒体电子传输链的抑制作用的测量。一项研究发现,鱼藤酮和异烟肼的组合分别抑制线粒体复合体I和II,在体外引起肝细胞毒性方面具有协同作用,并建议与另一种抑制复合物I的药物合用会增加异烟肼引起的患者肝损伤的风险。我们在体内测试了这一假设,在野生型和PD-1 – / –小鼠中,我们的免疫耐受性受损的小鼠模型抗CTLA-4被给予了水中0.02%(w / v)的鱼藤酮或0.1%,0.05% ,以及食品中异烟肼的0.01%(w / w)鱼藤酮。共同处理导致100%的动物接受0.1%鱼藤酮和0.2%异烟肼的致死性,83%的动物与食物中的0.05%鱼藤酮和0.2%异烟肼共同处理。然而,GLDH或肝损伤的组织学证据没有显着增加。在单独给予鱼藤酮或异烟肼的任何小鼠中均未观察到毒性迹象。即使抑制线粒体电子运输链不会导致明显的肝毒性,它可能会提供危险信号,促进免疫介导的肝损伤。但是,在我们受损的免疫耐受模型中,鱼藤酮并未显着增加异烟肼引起的肝损伤。总的来说,我们得出的结论是,抑制线粒体电子传输链不是IDILI的重要机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号