当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coexisting Few-Layer Assemblies of NiO and MoO3 Deposited on Vulcan Carbon as an Efficient and Durable Electrocatalyst for Water Oxidation
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-06-18 00:00:00 , DOI: 10.1021/acsaem.9b00665 Rajith Illathvalappil 1, 2 , Leena George 2, 3 , Sreekumar Kurungot 1, 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-06-18 00:00:00 , DOI: 10.1021/acsaem.9b00665 Rajith Illathvalappil 1, 2 , Leena George 2, 3 , Sreekumar Kurungot 1, 2
Affiliation
|
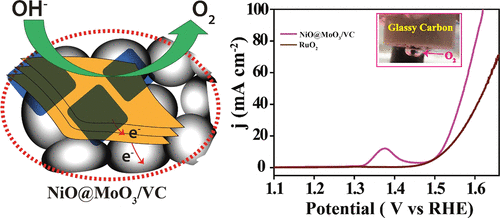
An efficient electrocatalyst for oxygen evolution reaction (OER) has been prepared by adopting a strategy wherein layered assembly of NiO and MoO3 could be dispersed on a Vulcan carbon support to simultaneously maintain exposure of the synergistically activated sites and electrical conductivity of the matrix. The assembly involves growth of layers of NiO on the surface of few-layer MoO3, which in turn is dispersed on Vulcan carbon ([email protected]3/VC) through a sequential hydrothermal process. The hydrothermal process played a key role in establishing the coexisting few-layer assembly of NiO and MoO3 on Vulcan carbon, which involved conversion of preformed MoS2 sheets to MoO3 and concomitant growth of Ni(OH)2 layer prior to its conversion to NiO during the subsequent heat treatment process. The NiO sheets have exposed edges, and they are instrumental in enhancing the OER activity of [email protected]3/VC. The higher activity is also credited to the lower charge transfer resistance (RCT) value possessed by [email protected]3/VC, which arises from the optimum combination of MoO3 and Vulcan carbon in the system. The overpotential of [email protected]3/VC for achieving the 10 mA cm–2 current density is 280 mV, which is an improved value over the 292 mV obtained in the case of the state-of-the-art RuO2 catalyst. [email protected]3/VC also demonstrates outstanding stability for 15 h in 1 M KOH solution at 1.51 V. The presence of surface nickel molybdate (NiMoO4) prevents the dissolution of MoO3 in the alkaline environment and provides excellent stability to the [email protected]3/VC in 1 M KOH solution.
中文翻译:

作为高效且耐用的水氧化电催化剂,在火神碳上沉积的少量NiO和MoO 3共存
通过采用一种策略,可以将NiO和MoO 3的分层组件分散在Vulcan碳载体上,以同时维持协同活化位点的暴露和基质的电导率,从而制备出一种高效的氧释放反应电催化剂(OER)。该组装涉及在少数MoO 3的表面上生长NiO层,然后依次通过水热过程将其分散在Vulcan碳([受电子邮件保护] 3 / VC)上。水热过程在建立Vulcan碳上NiO和MoO 3共存的多层组装中起着关键作用,这涉及将预制的MoS 2片材转化为MoO 3Ni(OH)2层在随后的热处理过程中转变为NiO之前伴随生长。NiO薄板具有暴露的边缘,它们有助于增强[受电子邮件保护的] 3 / VC的OER活性。较高的活性也归因于[电子邮件保护] 3 / VC具有较低的电荷转移阻力(R CT)值,这是由于系统中MoO 3和Vulcan碳的最佳组合引起的。[电子邮件保护的] 3 / VC达到10 mA cm –2的电流密度的过电势为280 mV,这是在最先进的RuO 2情况下获得的292 mV的改进值催化剂。[受电子邮件保护] 3 / VC在1M KOH溶液中于1.51 V电压下也显示出15个小时的出色稳定性。表面钼酸镍(NiMoO 4)的存在可防止MoO 3在碱性环境中的溶解,并为[[电子邮件保护] 1 M KOH解决方案中的3 / VC。
更新日期:2019-06-18
中文翻译:

作为高效且耐用的水氧化电催化剂,在火神碳上沉积的少量NiO和MoO 3共存
通过采用一种策略,可以将NiO和MoO 3的分层组件分散在Vulcan碳载体上,以同时维持协同活化位点的暴露和基质的电导率,从而制备出一种高效的氧释放反应电催化剂(OER)。该组装涉及在少数MoO 3的表面上生长NiO层,然后依次通过水热过程将其分散在Vulcan碳([受电子邮件保护] 3 / VC)上。水热过程在建立Vulcan碳上NiO和MoO 3共存的多层组装中起着关键作用,这涉及将预制的MoS 2片材转化为MoO 3Ni(OH)2层在随后的热处理过程中转变为NiO之前伴随生长。NiO薄板具有暴露的边缘,它们有助于增强[受电子邮件保护的] 3 / VC的OER活性。较高的活性也归因于[电子邮件保护] 3 / VC具有较低的电荷转移阻力(R CT)值,这是由于系统中MoO 3和Vulcan碳的最佳组合引起的。[电子邮件保护的] 3 / VC达到10 mA cm –2的电流密度的过电势为280 mV,这是在最先进的RuO 2情况下获得的292 mV的改进值催化剂。[受电子邮件保护] 3 / VC在1M KOH溶液中于1.51 V电压下也显示出15个小时的出色稳定性。表面钼酸镍(NiMoO 4)的存在可防止MoO 3在碱性环境中的溶解,并为[[电子邮件保护] 1 M KOH解决方案中的3 / VC。































 京公网安备 11010802027423号
京公网安备 11010802027423号