当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Dihydronaphthalene‐1,4‐Diones via Carbene‐Catalyzed Stereodivergent Reaction
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-07-11 , DOI: 10.1002/adsc.201900506 Zhifei Zhao 1 , Shuang Yang 1 , Shouang Lan 1 , Jinggong Liu 2 , Shuhua Liu 3 , Xinqiang Fang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-07-11 , DOI: 10.1002/adsc.201900506 Zhifei Zhao 1 , Shuang Yang 1 , Shouang Lan 1 , Jinggong Liu 2 , Shuhua Liu 3 , Xinqiang Fang 1
Affiliation
|
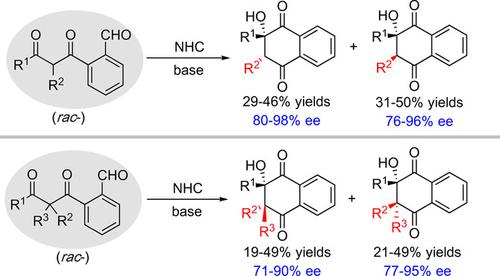
2‐Hydroxy‐2,3‐dihydronaphthalene‐1,4‐diones (HDNDs) are ubiquitous in natural products and bioactive molecules, but the rapid asymmetric construction of such scaffolds remains a significant challenge to date. Reported herein is the rapid construction of the above key units via carbene‐catalyzed benzoin reaction. The resolution technique of divergent reaction on racemic mixture (divergent RRM) was employed, affording both isomers of HDNDs in a one‐step fashion. Disubstituted substrates afford products with two contiguous quaternary stereocenters. A series of highly selective transformations on the products can be realized, and mechanistic studies indicate that the benzoin reaction is much faster than the racemization process and the aldol reaction.
中文翻译:

碳催化立体发散反应的不对称合成二氢萘-1,4-二酮
2-羟基-2,3-二氢萘-1,4-二酮(HDNDs)在天然产物和生物活性分子中无处不在,但是迄今为止,这种支架的快速不对称结构仍然是一个重大挑战。本文报道的是通过卡宾催化的安息香反应快速构建上述关键单元。采用了在外消旋混合物上发散反应的拆分技术(发散的RRM),一步一步提供了HDNDs的两种异构体。双取代的底物提供具有两个连续的四级立体中心的产物。可以实现对产物的一系列高度选择性的转化,并且机理研究表明,安息香反应比消旋过程和醛醇缩合反应快得多。
更新日期:2019-07-11
中文翻译:

碳催化立体发散反应的不对称合成二氢萘-1,4-二酮
2-羟基-2,3-二氢萘-1,4-二酮(HDNDs)在天然产物和生物活性分子中无处不在,但是迄今为止,这种支架的快速不对称结构仍然是一个重大挑战。本文报道的是通过卡宾催化的安息香反应快速构建上述关键单元。采用了在外消旋混合物上发散反应的拆分技术(发散的RRM),一步一步提供了HDNDs的两种异构体。双取代的底物提供具有两个连续的四级立体中心的产物。可以实现对产物的一系列高度选择性的转化,并且机理研究表明,安息香反应比消旋过程和醛醇缩合反应快得多。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号