当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NIR-Activated Spatiotemporally Controllable Nanoagent for Achieving Synergistic Gene-Chemo-Photothermal Therapy in Tumor Ablation
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2019-06-25 , DOI: 10.1021/acsabm.9b00329 Xue-Jiao Yang 1 , Xiang-Ling Li 1 , Hong-Yuan Chen 1 , Jing-Juan Xu 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2019-06-25 , DOI: 10.1021/acsabm.9b00329 Xue-Jiao Yang 1 , Xiang-Ling Li 1 , Hong-Yuan Chen 1 , Jing-Juan Xu 1
Affiliation
|
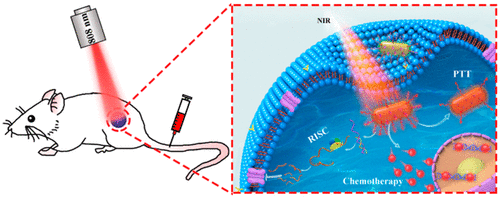
A rational combination of different therapeutic modalities within one single nanostructure is promising to enhance the therapeutic response, especially to achieve a synergistic therapeutic efficacy for tumor treatment. Herein, a near-infrared (NIR) photothermally activated nanoagent, which could achieve a spatially controllable codelivery of different curative molecules and a temporally controlled responsive release, was designed to perform effective gene-chemo-photothermal therapy of malignant tumors. The nanoagent consisted of a gold nanorod (AuNR) functionalized with mPEG, DNA, and small interfering RNA (siRNA). With the aid of aptamer AS1411-mediated recognition and endocytosis, the nanoagents were selectively delivered into cancer cells; subsequently, the photothermal conversion of AuNRs happened while under NIR irradiation, which successfully achieved an effective photothermal therapy, induced dehybridization of DNA duplexes, and simultaneously released doxorubicin (DOX) and siRNA. Then, the released siRNA silenced the expression of the multidrug resistance associated protein 1 (MRP1), the primary cause of the undesirable expelling of DOX in PC-3 cells, yielding a remarkable improvement in the efficiency of gene-chemo therapy. All of the results of in vitro and in vivo studies revealed that our prepared nanoagents exhibited an excellent performance in synergistic gene-chemo-photothermal therapy and successfully inhibited tumor growth. This work provides an interesting concept of a nanoscale therapeutic agent in achieving a dramatically enhanced therapeutic ability for tumor ablation, which benefits from the spatiotemporally controllable properties and the synergistic combination of chemotherapy, gene therapy, and photothermal therapy in one single nanoagent.
中文翻译:

近红外激活时空可控纳米剂在肿瘤消融中实现协同基因-化学-光热治疗
在一个单一的纳米结构内合理组合不同的治疗方式有望增强治疗反应,尤其是实现肿瘤治疗的协同治疗效果。在此,一种近红外(NIR)光热激活纳米剂可以实现不同治疗分子的空间可控协同传递和时间可控的响应释放,旨在对恶性肿瘤进行有效的基因-化学-光热治疗。该纳米剂由一个金纳米棒 (AuNR) 组成,该纳米棒用 mPEG、DNA 和小干扰 RNA (siRNA) 进行了功能化。借助适体 AS1411 介导的识别和内吞作用,将纳米药剂选择性地递送到癌细胞中;随后,AuNRs 的光热转化发生在 NIR 照射下,成功实现了有效的光热疗法,诱导DNA双链体去杂交,同时释放多柔比星(DOX)和siRNA。然后,释放的 siRNA 沉默了多药耐药相关蛋白 1 (MRP1) 的表达,这是 PC-3 细胞中 DOX 不良排出的主要原因,显着提高了基因化疗的效率。的所有结果体外和体内研究表明,我们制备的纳米制剂在协同基因-化学-光热疗法中表现出优异的性能,并成功地抑制了肿瘤的生长。这项工作提供了一个有趣的概念,即纳米级治疗剂在实现显着增强的肿瘤消融治疗能力方面,这得益于单一纳米剂中的时空可控特性和化学疗法、基因疗法和光热疗法的协同组合。
更新日期:2019-06-26
中文翻译:

近红外激活时空可控纳米剂在肿瘤消融中实现协同基因-化学-光热治疗
在一个单一的纳米结构内合理组合不同的治疗方式有望增强治疗反应,尤其是实现肿瘤治疗的协同治疗效果。在此,一种近红外(NIR)光热激活纳米剂可以实现不同治疗分子的空间可控协同传递和时间可控的响应释放,旨在对恶性肿瘤进行有效的基因-化学-光热治疗。该纳米剂由一个金纳米棒 (AuNR) 组成,该纳米棒用 mPEG、DNA 和小干扰 RNA (siRNA) 进行了功能化。借助适体 AS1411 介导的识别和内吞作用,将纳米药剂选择性地递送到癌细胞中;随后,AuNRs 的光热转化发生在 NIR 照射下,成功实现了有效的光热疗法,诱导DNA双链体去杂交,同时释放多柔比星(DOX)和siRNA。然后,释放的 siRNA 沉默了多药耐药相关蛋白 1 (MRP1) 的表达,这是 PC-3 细胞中 DOX 不良排出的主要原因,显着提高了基因化疗的效率。的所有结果体外和体内研究表明,我们制备的纳米制剂在协同基因-化学-光热疗法中表现出优异的性能,并成功地抑制了肿瘤的生长。这项工作提供了一个有趣的概念,即纳米级治疗剂在实现显着增强的肿瘤消融治疗能力方面,这得益于单一纳米剂中的时空可控特性和化学疗法、基因疗法和光热疗法的协同组合。






























 京公网安备 11010802027423号
京公网安备 11010802027423号