当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pt/TiH2 Catalyst for Ionic Hydrogenation via Stored Hydrides in the Presence of Gaseous H2
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-06-14 00:00:00 , DOI: 10.1021/acscatal.9b00917 Qifan Wu 1, 2, 3 , Chao Zhang 1, 3 , Masahiko Arai 1, 3 , Bin Zhang 1, 2, 3 , Ruhui Shi 1, 2, 3 , Peixuan Wu 1, 2, 3 , Zhuangqing Wang 1, 2, 3 , Qiang Liu 1, 3 , Ke Liu 1, 3 , Weiwei Lin 1, 3 , Haiyang Cheng 1, 3 , Fengyu Zhao 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-06-14 00:00:00 , DOI: 10.1021/acscatal.9b00917 Qifan Wu 1, 2, 3 , Chao Zhang 1, 3 , Masahiko Arai 1, 3 , Bin Zhang 1, 2, 3 , Ruhui Shi 1, 2, 3 , Peixuan Wu 1, 2, 3 , Zhuangqing Wang 1, 2, 3 , Qiang Liu 1, 3 , Ke Liu 1, 3 , Weiwei Lin 1, 3 , Haiyang Cheng 1, 3 , Fengyu Zhao 1, 3
Affiliation
|
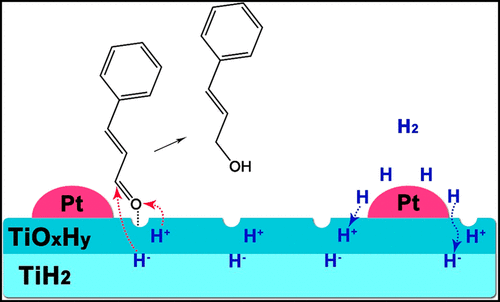
A Pt/TiH2 catalyst having hydrogen storage and release capability was investigated for selective hydrogenation of trans-cinnamaldehyde (CAL) to cinnamyl alcohol (COL) with gaseous dihydrogen. The catalytic behavior of this catalyst was significantly different from that of a reference Pt/TiO2 catalyst with respect to the product selectivity and the hydrogenation mechanism. The Pt/TiH2 catalyst showed a COL selectivity of 97% at a CAL conversion of 98%, which was ascribed to the function of a Pt crystallite–support boundary layer that caused the preferential adsorption of CAL with its carbonyl group. Furthermore, the carbonyl group was hydrogenated by hydride species (H+, H–) supplied from the support and the hydride species consumed were compensated from gaseous dihydrogen; hydrogen atoms were formed by ordinary homolytic cleavage on Pt and then these hydrogen atoms moved onto the surface of TiH2 and diffused into the bulk of the support, during which those simultaneously changed to hydride species (H+, H–) via electron transfer with titanium species and hydride vacancies therein. The surface and bulk diffusion of the hydrogen atoms from Pt to TiH2 support should be the dominant step rather than their addition to the carbonyl group of CAL (ordinary hydrogenation). That is, ionic hydrogenation occurs with Pt/TiH2 in the presence of gaseous dihydrogen.
中文翻译:

在气态H 2存在下通过储存的氢化物进行离子加氢的Pt / TiH 2催化剂
的Pt / TIH 2催化剂具有氢的储存和释放能力进行了研究用于选择性氢化反-cinnamaldehyde(CAL)到肉桂醇(COL)与气态二氢。就产物选择性和氢化机理而言,该催化剂的催化行为与参考Pt / TiO 2催化剂明显不同。Pt / TiH 2催化剂在CAL转化率为98%时显示出97%的COL选择性,这归因于Pt晶体-载体边界层的功能,该边界层导致CAL优先被其羰基吸附。此外,羰基的氢化物物种(H氢化+,H -)从载体中提供,消耗的氢化物通过气态二氢得到补偿;的氢原子被普通均裂在Pt形成,然后将这些氢原子移动到载通的表面2和扩散到本体的支持,在此期间,那些同时改变到氢化物种(H +,H - )经由电子转移与钛物种和其中的氢化物空位。氢原子从Pt到TiH 2载体的表面和整体扩散应该是主要步骤,而不是将它们加到CAL的羰基上(常规氢化)。即,在气态二氢存在下,Pt / TiH 2发生离子氢化。
更新日期:2019-06-14
中文翻译:

在气态H 2存在下通过储存的氢化物进行离子加氢的Pt / TiH 2催化剂
的Pt / TIH 2催化剂具有氢的储存和释放能力进行了研究用于选择性氢化反-cinnamaldehyde(CAL)到肉桂醇(COL)与气态二氢。就产物选择性和氢化机理而言,该催化剂的催化行为与参考Pt / TiO 2催化剂明显不同。Pt / TiH 2催化剂在CAL转化率为98%时显示出97%的COL选择性,这归因于Pt晶体-载体边界层的功能,该边界层导致CAL优先被其羰基吸附。此外,羰基的氢化物物种(H氢化+,H -)从载体中提供,消耗的氢化物通过气态二氢得到补偿;的氢原子被普通均裂在Pt形成,然后将这些氢原子移动到载通的表面2和扩散到本体的支持,在此期间,那些同时改变到氢化物种(H +,H - )经由电子转移与钛物种和其中的氢化物空位。氢原子从Pt到TiH 2载体的表面和整体扩散应该是主要步骤,而不是将它们加到CAL的羰基上(常规氢化)。即,在气态二氢存在下,Pt / TiH 2发生离子氢化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号