当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting the Cleavage of Evans Oxazolidinones with LiOH/H2O2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-06-16 00:00:00 , DOI: 10.1021/acs.oprd.9b00124 Gregory L. Beutner 1 , Benjamin M. Cohen 1 , Albert J. DelMonte 1 , Darryl D. Dixon 1 , Kenneth J. Fraunhoffer 1 , Andrew W. Glace 1 , Ehrlic Lo 1 , Jason M. Stevens 1 , Dale Vanyo 1 , Christopher Wilbert 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-06-16 00:00:00 , DOI: 10.1021/acs.oprd.9b00124 Gregory L. Beutner 1 , Benjamin M. Cohen 1 , Albert J. DelMonte 1 , Darryl D. Dixon 1 , Kenneth J. Fraunhoffer 1 , Andrew W. Glace 1 , Ehrlic Lo 1 , Jason M. Stevens 1 , Dale Vanyo 1 , Christopher Wilbert 1
Affiliation
|
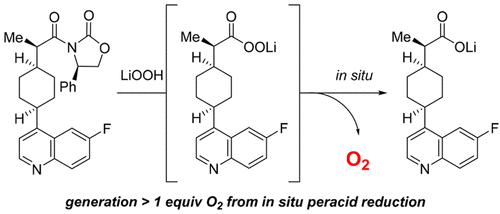
The use of LiOH/H2O2 for cleavage of Evans oxazolidinones is an essential but often overlooked part of using these ubiquitous chiral auxiliaries in synthetic routes. These conditions were disclosed in the literature more than 30 years ago and have found widespread use with little change. During studies focused on the synthesis of a drug candidate, we discovered the evolution of oxygen under the LiOH/H2O2 conditions for cleavage of an Evans auxiliary. We find this phenomenon to be general and tied to the fact that the initially formed peracid is not stable under the reaction conditions. The peracid is rapidly reduced by the excess H2O2 present in the reaction mixture, leading to the release of a stoichiometric amount of oxygen. This can present a significant risk for maintaining proper inertion in the presence of the flammable organic solvent, and we propose options for safely executing this chemistry at multikilogram scale.
中文翻译:

用LiOH / H 2 O 2考察伊万斯恶唑烷酮的裂解
LiOH / H 2 O 2用于裂解伊万恶唑烷酮是在合成路线中使用这些无处不在的手性助剂的必不可少的但经常被忽略的部分。这些条件已在30多年前的文献中披露,并且已发现其广泛使用而几乎没有变化。在专注于候选药物合成的研究过程中,我们发现了在LiOH / H 2 O 2条件下氧的逸出以裂解Evans助剂。我们发现该现象是普遍现象,并且与以下事实有关:在反应条件下,最初形成的过酸不稳定。过酸通过过量的H 2 O 2迅速还原存在于反应混合物中,导致释放出化学计量的氧气。在存在易燃有机溶剂的情况下,这可能会带来维持适当惰性的巨大风险,因此,我们提出了以多千克为单位安全执行此化学反应的选项。
更新日期:2019-06-16
中文翻译:

用LiOH / H 2 O 2考察伊万斯恶唑烷酮的裂解
LiOH / H 2 O 2用于裂解伊万恶唑烷酮是在合成路线中使用这些无处不在的手性助剂的必不可少的但经常被忽略的部分。这些条件已在30多年前的文献中披露,并且已发现其广泛使用而几乎没有变化。在专注于候选药物合成的研究过程中,我们发现了在LiOH / H 2 O 2条件下氧的逸出以裂解Evans助剂。我们发现该现象是普遍现象,并且与以下事实有关:在反应条件下,最初形成的过酸不稳定。过酸通过过量的H 2 O 2迅速还原存在于反应混合物中,导致释放出化学计量的氧气。在存在易燃有机溶剂的情况下,这可能会带来维持适当惰性的巨大风险,因此,我们提出了以多千克为单位安全执行此化学反应的选项。













































 京公网安备 11010802027423号
京公网安备 11010802027423号