Nature Communications ( IF 14.7 ) Pub Date : 2019-06-14 , DOI: 10.1038/s41467-019-10512-6 Xiaodong Shi 1, 2 , Muyuan Chen 1 , Zhili Yu 1 , James M Bell 1, 3 , Hans Wang 1 , Isaac Forrester 4 , Heather Villarreal 4 , Joanita Jakana 4 , Dijun Du 5, 6 , Ben F Luisi 5 , Steven J Ludtke 1 , Zhao Wang 1, 7
|
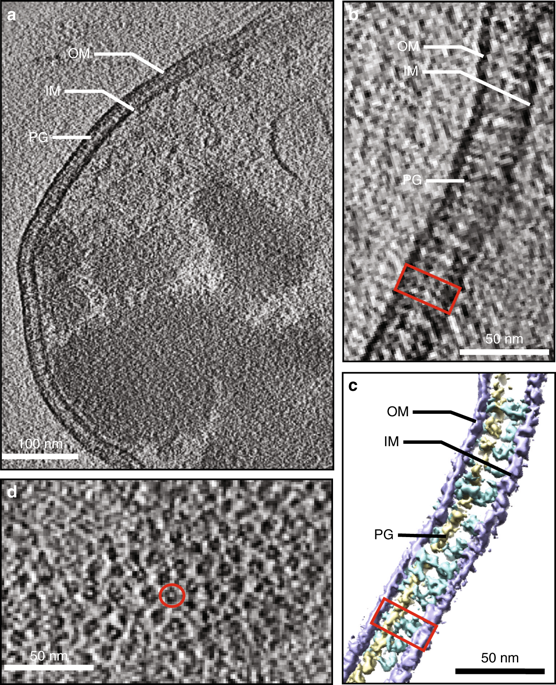
Multidrug efflux pumps actively expel a wide range of toxic substrates from the cell and play a major role in intrinsic and acquired drug resistance. In Gram-negative bacteria, these pumps form tripartite assemblies that span the cell envelope. However, the in situ structure and assembly mechanism of multidrug efflux pumps remain unknown. Here we report the in situ structure of the Escherichia coli AcrAB-TolC multidrug efflux pump obtained by electron cryo-tomography and subtomogram averaging. The fully assembled efflux pump is observed in a closed state under conditions of antibiotic challenge and in an open state in the presence of AcrB inhibitor. We also observe intermediate AcrAB complexes without TolC and discover that AcrA contacts the peptidoglycan layer of the periplasm. Our data point to a sequential assembly process in living bacteria, beginning with formation of the AcrAB subcomplex and suggest domains to target with efflux pump inhibitors.
中文翻译:

多药外排泵AcrAB-TolC的原位结构和组装。
Multidrug外排泵可从细胞中积极排出多种有毒底物,并在固有和获得性耐药中发挥重要作用。在革兰氏阴性细菌中,这些泵形成跨细胞包膜的三方组件。然而,多药外排泵的原位结构和组装机理仍然未知。在这里,我们报告了大肠杆菌的原位结构AcrAB-TolC多药外排泵,通过电子冷冻断层摄影和亚断层摄影平均法获得。在抗生素攻击的条件下,在关闭状态下观察到完全组装的外排泵,在存在AcrB抑制剂的情况下,在打开状态下观察到。我们还观察了没有TolC的中间AcrAB复合物,发现AcrA与周质的肽聚糖层接触。我们的数据指出了从AcrAB亚复合物的形成开始,在活细菌中的顺序组装过程,并提出了以外排泵抑制剂为靶标的结构域。






























 京公网安备 11010802027423号
京公网安备 11010802027423号