Communications Chemistry ( IF 5.9 ) Pub Date : 2019-06-14 , DOI: 10.1038/s42004-019-0168-6 Shuaipeng Lv , Hui Zhou , Xin Yu , Yue Xu , Huijuan Zhu , Min Wang , Haitao Liu , Ziru Dai , Guibo Sun , Xiaojie Gong , Xiaobo Sun , Lei Wang
|
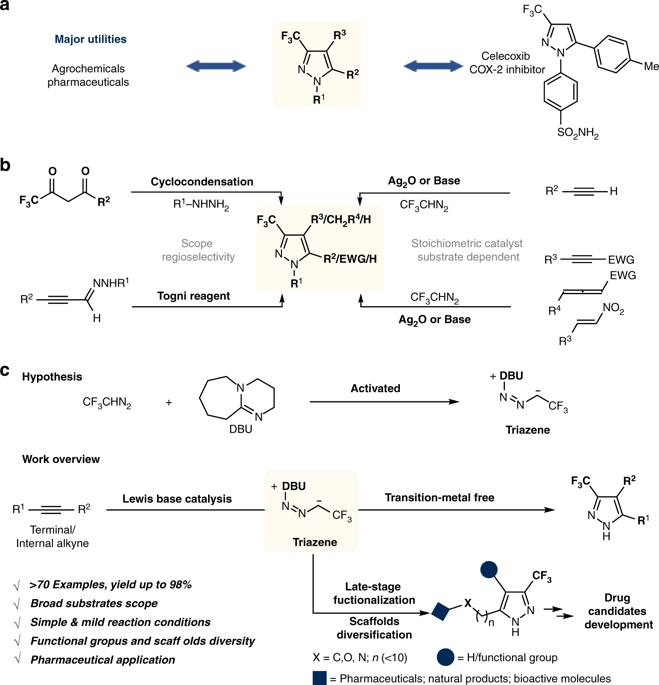
3-Trifluoromethylpyrazole and its derivatives are of major interest to both the agrochemical and pharmaceutical industry for their diverse biological activities. Reported routes for the synthesis of 3-trifluoromethylpyrazoles are hindered by poor regioselectivity and limited scope of application. Here we report a directed Lewis base catalyzed intermolecular triazene-alkyne cycloaddition. It is featured that the combination of 1,8-diazabicyclo[5.4.0]undec-7-ene and 2,2,2-trifluorodiazoethane produces reactive triazene intermediates, which readily participate in cycloaddition reactions with terminal/internal alkynes, thus assembling densely substituted 3-trifluoromethylpyrazole scaffolds with environmental friendliness and operational simplicity. Synthetic utility of the protocol is highlighted by late-stage functionalization and scaffolds diversification. The practical value is also emphasized in potential platelet aggregation inhibitor synthesis.
中文翻译:

Lewis碱催化的分子间三氮烯炔烃环加成反应,用于后期功能化和支架多样化
3-三氟甲基吡唑及其衍生物因其多种生物活性而受到农业化学和制药工业的广泛关注。报道的3-三氟甲基吡唑的合成路线受到不良的区域选择性和有限的应用范围的阻碍。在这里,我们报告定向的路易斯碱催化的分子间三氮烯-炔环加成反应。其特征在于1,8-二氮杂双环[5.4.0]十一碳-7-烯和2,2,2-三氟重氮乙烷的结合产生反应性三氮烯中间体,该中间体容易参与与末端/内部炔烃的环加成反应,从而紧密地组装具有环境友好性和操作简便性的取代3-三氟甲基吡唑支架。该协议的合成实用性在后期功能化和支架多样化中得到了强调。在潜在的血小板凝集抑制剂合成中也强调了实用价值。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号