当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Variations of 1H NMR Chemical Shifts in Benzene Substituted with an Electron‐Accepting (NO2)/Donating (NH2) Group be Explained in Terms of Resonance Effects of Substituents?
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-03-06 , DOI: 10.1002/asia.201800137 Marija Baranac-Stojanović 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-03-06 , DOI: 10.1002/asia.201800137 Marija Baranac-Stojanović 1
Affiliation
|
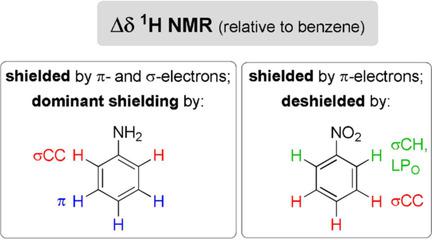
The classical textbook explanation of variations of 1H NMR chemical shifts in benzenes bearing an electron‐donating (NH2) or an electron‐withdrawing (NO2) group in terms of substituent resonance effects was examined by analyzing molecular orbital contributions to the total shielding. It was found that the π‐electronic system showed a more pronounced shielding effect on all ring hydrogen atoms, relative to benzene, irrespective of substituent +R/−R effects. For the latter, this was in contrast to the traditional explanations of downfield shift of nitrobenzene proton resonances, which were found to be determined by the σ‐electronic system and oxygen in‐plane lone pairs. In aniline, the +R effect of NH2 group can be used to fully explain the upfield position of meta‐H signals and partly the upfield position of para‐H signals, the latter also being influenced by the σ‐system. The position of the lowest frequency signal of ortho‐Hs was fully determined by σ‐electrons.
中文翻译:

可以用取代基的共振效应来解释用电子接受(NO2)/取代键(NH2)取代的苯中1H NMR化学位移的变化吗?
通过分析分子轨道对总屏蔽作用的影响,研究了经典教科书对带有给电子基团(NH 2)或吸电子基团(NO 2)的苯中1 H NMR化学位移变化的解释。。已发现,相对于苯,π电子系统对所有环氢原子均表现出更显着的屏蔽效应,而与取代基+ R / -R效应无关。对于后者,这与对硝基苯质子共振的场下位移的传统解释相反,后者是由σ电子系统和氧平面内孤对决定的。在苯胺中,NH 2的+ R效应小组可以用来全面解释meta- H信号的高场位置,部分解释para- H信号的高场位置,后者也受到σ系统的影响。邻-Hs最低频率信号的位置完全由σ-电子确定。
更新日期:2018-03-06
中文翻译:

可以用取代基的共振效应来解释用电子接受(NO2)/取代键(NH2)取代的苯中1H NMR化学位移的变化吗?
通过分析分子轨道对总屏蔽作用的影响,研究了经典教科书对带有给电子基团(NH 2)或吸电子基团(NO 2)的苯中1 H NMR化学位移变化的解释。。已发现,相对于苯,π电子系统对所有环氢原子均表现出更显着的屏蔽效应,而与取代基+ R / -R效应无关。对于后者,这与对硝基苯质子共振的场下位移的传统解释相反,后者是由σ电子系统和氧平面内孤对决定的。在苯胺中,NH 2的+ R效应小组可以用来全面解释meta- H信号的高场位置,部分解释para- H信号的高场位置,后者也受到σ系统的影响。邻-Hs最低频率信号的位置完全由σ-电子确定。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号