当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery and Biological evaluation of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione derivatives as potent Bruton's tyrosine kinase inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-06-12 , DOI: 10.1016/j.bmc.2019.06.023 Yanyan Diao 1 , Xiaoyu Fang 1 , Peiran Song 2 , Mengzhen Lai 3 , Linjiang Tong 4 , Yongjia Hao 1 , Dou Dou 1 , Yingqiang Liu 4 , Jian Ding 2 , Zhenjiang Zhao 1 , Hua Xie 4 , Honglin Li 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-06-12 , DOI: 10.1016/j.bmc.2019.06.023 Yanyan Diao 1 , Xiaoyu Fang 1 , Peiran Song 2 , Mengzhen Lai 3 , Linjiang Tong 4 , Yongjia Hao 1 , Dou Dou 1 , Yingqiang Liu 4 , Jian Ding 2 , Zhenjiang Zhao 1 , Hua Xie 4 , Honglin Li 1
Affiliation
|
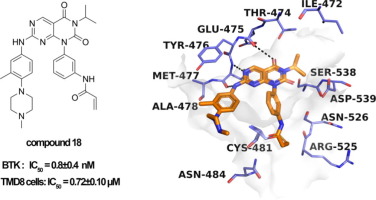
Aberrant activation of B cell receptor (BCR) signal transduction cascade contributes to the propagation and maintenance of B cell malignancies. The discovery of mall molecules with high potency and selectivity against Bruton's tyrosine kinase (BTK), a key signaling molecule in this cascade, is particularly urgent in modern treatment regimens. Herein, a series of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione derivatives were reported as potent BTK inhibitors. Compounds 17 and 18 displayed strong BTK inhibitory activities in the enzymatic inhibition assay, with the IC50 values of 1.2 and 0.8 nM, respectively, which were comparable to that of ibrutinib (IC50 = 0.6 nM). Additionally, compound 17 had a more selective profile over EGFR than ibrutinib. According to the putative binding poses, the molecular basis of this series of compounds with respect to potency against BTK and selectivity over EGFR was elucidated. In further experiments at cellular level, compounds 17 and 18 significantly inhibited the proliferation of Ramos and TMD8 cells. And they arrested 75.4% and 75.2% of TMD8 cells in G1 phase, respectively, at the concentration of 1 µM.
中文翻译:

作为有效的布鲁顿酪氨酸激酶抑制剂的嘧啶并[4,5-d]嘧啶-2,4(1H,3H)-二酮衍生物的发现和生物学评估。
B细胞受体(BCR)信号转导级联的异常激活有助于B细胞恶性肿瘤的传播和维持。在现代治疗方案中,发现针对这种级联反应中的关键信号分子布鲁顿酪氨酸激酶(BTK)具有高效力和选择性的商城分子非常重要。在此,据报道一系列嘧啶[4,5-d]嘧啶-2,4(1H,3H)-二酮衍生物是有效的BTK抑制剂。化合物17和18在酶促抑制试验中显示出强大的BTK抑制活性,IC50值分别为1.2和0.8 nM,与依鲁替尼相当(IC50 = 0.6 nM)。此外,与依鲁替尼相比,化合物17对EGFR的选择性更高。根据假定的约束姿势,阐明了该系列化合物相对于BTK的效力和对EGFR的选择性的分子基础。在细胞水平的进一步实验中,化合物17和18显着抑制Ramos和TMD8细胞的增殖。他们以1 µM的浓度分别捕获了G1期的75.4%和75.2%的TMD8细胞。
更新日期:2019-06-12
中文翻译:

作为有效的布鲁顿酪氨酸激酶抑制剂的嘧啶并[4,5-d]嘧啶-2,4(1H,3H)-二酮衍生物的发现和生物学评估。
B细胞受体(BCR)信号转导级联的异常激活有助于B细胞恶性肿瘤的传播和维持。在现代治疗方案中,发现针对这种级联反应中的关键信号分子布鲁顿酪氨酸激酶(BTK)具有高效力和选择性的商城分子非常重要。在此,据报道一系列嘧啶[4,5-d]嘧啶-2,4(1H,3H)-二酮衍生物是有效的BTK抑制剂。化合物17和18在酶促抑制试验中显示出强大的BTK抑制活性,IC50值分别为1.2和0.8 nM,与依鲁替尼相当(IC50 = 0.6 nM)。此外,与依鲁替尼相比,化合物17对EGFR的选择性更高。根据假定的约束姿势,阐明了该系列化合物相对于BTK的效力和对EGFR的选择性的分子基础。在细胞水平的进一步实验中,化合物17和18显着抑制Ramos和TMD8细胞的增殖。他们以1 µM的浓度分别捕获了G1期的75.4%和75.2%的TMD8细胞。






































 京公网安备 11010802027423号
京公网安备 11010802027423号