当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling microcephaly with cerebral organoids reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly in neural progenitors.
Nature Communications ( IF 14.7 ) Pub Date : 2019-06-13 , DOI: 10.1038/s41467-019-10497-2
Wei Zhang 1 , Si-Lu Yang 2 , Mei Yang 1 , Stephanie Herrlinger 2 , Qiang Shao 1 , John L Collar 2 , Edgar Fierro 2 , Yanhong Shi 3 , Aimin Liu 4 , Hui Lu 5 , Bruce E Herring 6 , Ming-Lei Guo 7 , Shilpa Buch 7 , Zhen Zhao 8 , Jian Xu 1 , Zhipeng Lu 9 , Jian-Fu Chen 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-06-13 , DOI: 10.1038/s41467-019-10497-2
Wei Zhang 1 , Si-Lu Yang 2 , Mei Yang 1 , Stephanie Herrlinger 2 , Qiang Shao 1 , John L Collar 2 , Edgar Fierro 2 , Yanhong Shi 3 , Aimin Liu 4 , Hui Lu 5 , Bruce E Herring 6 , Ming-Lei Guo 7 , Shilpa Buch 7 , Zhen Zhao 8 , Jian Xu 1 , Zhipeng Lu 9 , Jian-Fu Chen 1
Affiliation
|
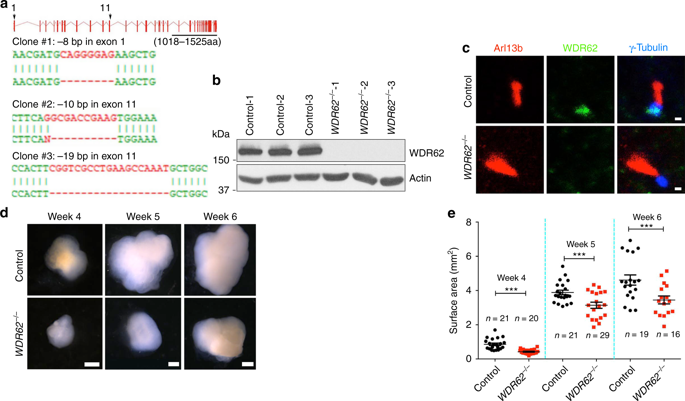
Primary microcephaly is caused by mutations in genes encoding centrosomal proteins including WDR62 and KIF2A. However, mechanisms underlying human microcephaly remain elusive. By creating mutant mice and human cerebral organoids, here we found that WDR62 deletion resulted in a reduction in the size of mouse brains and organoids due to the disruption of neural progenitor cells (NPCs), including outer radial glia (oRG). WDR62 ablation led to retarded cilium disassembly, long cilium, and delayed cell cycle progression leading to decreased proliferation and premature differentiation of NPCs. Mechanistically, WDR62 interacts with and promotes CEP170's localization to the basal body of primary cilium, where CEP170 recruits microtubule-depolymerizing factor KIF2A to disassemble cilium. WDR62 depletion reduced KIF2A's basal body localization, and enhanced KIF2A expression partially rescued deficits in cilium length and NPC proliferation. Thus, modeling microcephaly with cerebral organoids and mice reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly, disruption of which contributes to microcephaly.
中文翻译:

用脑类器官模拟小头畸形,揭示了促进神经祖细胞纤毛分解的WDR62-CEP170-KIF2A途径。
原发性小头畸形是由编码中心体蛋白(包括WDR62和KIF2A)的基因突变引起的。但是,人类小头畸形的潜在机制仍然难以捉摸。通过创建突变小鼠和人类大脑类器官,我们发现WDR62缺失导致神经元祖细胞(NPC)的破坏,包括外神经胶质细胞(oRG)的出现,导致小鼠大脑和类器官的大小减少。WDR62消融可导致纤毛分解延迟,纤毛长和细胞周期进程延迟,从而导致NPC增殖减少和过早分化。从机制上讲,WDR62与CEP170相互作用并促进CEP170定位于初级纤毛的基体,在该处CEP170募集微管解聚因子KIF2A来分解纤毛。WDR62耗尽减少了KIF2A的基体定位,增强的KIF2A表达可部分挽救纤毛长度和NPC增殖的缺陷。因此,用大脑类器官和小鼠对小头畸形进行建模揭示了促进纤毛分解的WDR62-CEP170-KIF2A途径,其破坏导致小头畸形。
更新日期:2019-06-13
中文翻译:

用脑类器官模拟小头畸形,揭示了促进神经祖细胞纤毛分解的WDR62-CEP170-KIF2A途径。
原发性小头畸形是由编码中心体蛋白(包括WDR62和KIF2A)的基因突变引起的。但是,人类小头畸形的潜在机制仍然难以捉摸。通过创建突变小鼠和人类大脑类器官,我们发现WDR62缺失导致神经元祖细胞(NPC)的破坏,包括外神经胶质细胞(oRG)的出现,导致小鼠大脑和类器官的大小减少。WDR62消融可导致纤毛分解延迟,纤毛长和细胞周期进程延迟,从而导致NPC增殖减少和过早分化。从机制上讲,WDR62与CEP170相互作用并促进CEP170定位于初级纤毛的基体,在该处CEP170募集微管解聚因子KIF2A来分解纤毛。WDR62耗尽减少了KIF2A的基体定位,增强的KIF2A表达可部分挽救纤毛长度和NPC增殖的缺陷。因此,用大脑类器官和小鼠对小头畸形进行建模揭示了促进纤毛分解的WDR62-CEP170-KIF2A途径,其破坏导致小头畸形。

































 京公网安备 11010802027423号
京公网安备 11010802027423号