Polymer ( IF 4.1 ) Pub Date : 2019-06-12 , DOI: 10.1016/j.polymer.2019.121554 Eui-Soung Jang , Jovan Kamcev , Kentaro Kobayashi , Ni Yan , Rahul Sujanani , Theodore J. Dilenschneider , Ho Bum Park , Donald R. Paul , Benny D. Freeman
|
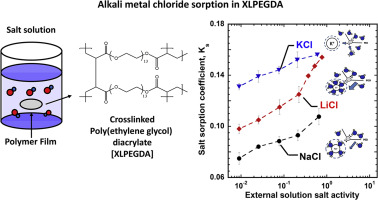
The relationship between ion size and sorption properties in water swollen uncharged polymers was investigated as a model system for understanding ion sorption and transport in more complex systems (i.e., charged polymer networks). Alkali metal chloride (e.g., LiCl, NaCl, and KCl) sorption coefficients in a series of cross-linked poly(ethylene glycol) diacrylate (XLPEGDA) polymer membranes were measured as a function of external salt concentrations ranging from 0.01 to 1.0 M. The relative order of salt sorption coefficients was: KCl > LiCl > NaCl. This order does not correspond to the order of ion hydration size (i.e., Li+ > Na+ > K+). The alkali metal chloride sorption behavior in XLPEGDA polymers is influenced by both ion hydration and polymer-ion specific interactions. XLPEGDA polymers having three different equilibrium water uptake values were prepared to investigate the effect of water content on salt sorption in these polymers. Generally, ion sorption coefficients increase as polymer water content increases. Salt activity coefficients in the polymers were quantified to better understand the thermodynamic non-ideality of ions in polymer networks. Flory-Rehner theory was used to predict water volume fraction in the polymers equilibrated with salt solution based on salt sorption measurements.
中文翻译:

水分含量对交联聚乙二醇二丙烯酸酯中碱金属氯化物传输的影响1。离子吸附
研究了水溶胀的不带电聚合物中离子尺寸与吸附性能之间的关系,作为一个模型系统来理解更复杂系统(即带电聚合物网络)中的离子吸附和迁移。测量了一系列交联的聚(乙二醇)二丙烯酸酯(XLPEGDA)聚合物膜中碱金属氯化物(例如LiCl,NaCl和KCl)的吸附系数,其作为外部盐浓度范围为0.01至1.0 M的函数。盐吸附系数的相对顺序为:KCl> LiCl> NaCl。此顺序与离子水合大小的顺序不符(即Li + > Na + > K +)。XLPEGDA聚合物中碱金属氯化物的吸附行为受离子水合和聚合物-离子特异性相互作用的影响。制备具有三种不同平衡吸水值的XLPEGDA聚合物,以研究水含量对这些聚合物中盐吸附的影响。通常,离子吸附系数随着聚合物水含量的增加而增加。对聚合物中的盐活度系数进行了定量,以更好地了解聚合物网络中离子的热力学非理想性。基于盐吸收测量,使用Flory-Rehner理论来预测用盐溶液平衡的聚合物中的水体积分数。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号