当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynein light chain binding determines complex formation and posttranslational stability of the Bcl-2 family members Bmf and Bim.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2019-06-12 , DOI: 10.1038/s41418-019-0365-y Prafull Kumar Singh 1 , Aristomenis Roukounakis 1 , Arnim Weber 1 , Kushal Kumar Das 2 , Benedicte Sohm 3, 4 , Andreas Villunger 3 , Ana J Garcia-Saez 2 , Georg Häcker 1, 5
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2019-06-12 , DOI: 10.1038/s41418-019-0365-y Prafull Kumar Singh 1 , Aristomenis Roukounakis 1 , Arnim Weber 1 , Kushal Kumar Das 2 , Benedicte Sohm 3, 4 , Andreas Villunger 3 , Ana J Garcia-Saez 2 , Georg Häcker 1, 5
Affiliation
|
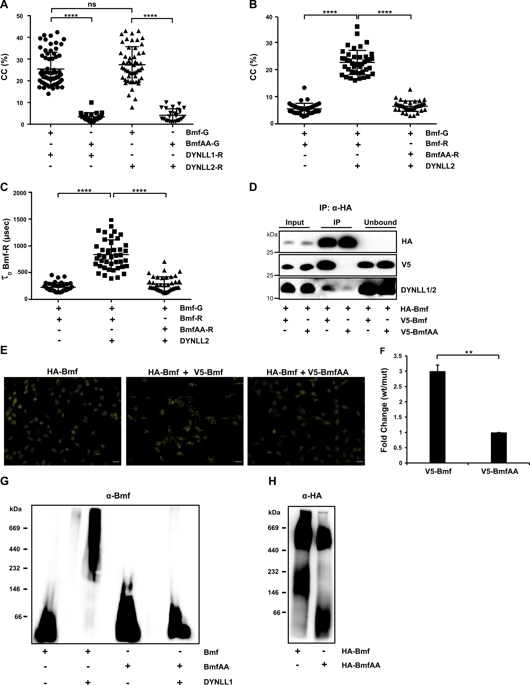
The BH3-only class of Bcl-2 family proteins triggers mitochondrial apoptosis. Several mechanisms are used to restrain the pro-apoptotic activity of these proteins. Dynein light chain (DYNLL) 1 and 2 has been proposed to negatively regulate the activity of Bim and Bmf, respectively, and the Bim-DYNLL1 interaction leads to the formation of large protein complexes on mitochondria. Here we found that Bim and Bmf interact with both isoforms of DYNLL (DYNLL1 and DYNLL2). DYNLL1/2 not only induced homo-dimerization of Bim and Bmf but also led to the formation of ternary complexes (Bim-DYNLL-Bmf), both in cell-free and in cellular systems. DYNLL-induced oligomerization stabilized Bmf in cultured cells and inhibited its degradation by the ubiquitin-independent 20S proteasome in a cell-free system. Surprisingly, overexpression of wild-type Bmf but not of a DYNLL-binding-deficient mutant induced degradation of endogenous Bim in different cell lines, but both variants sensitized to apoptosis. Mutant Bmf incapable of interacting with anti-apoptotic Bcl-2 proteins and of inducing apoptosis still caused Bim degradation. These results suggest that Bmf overexpression-induced Bim degradation is not due to the displacement of Bim from anti-apoptotic Bcl-2 proteins but a direct consequence of the modulation of Bim-DYNLL association. A peptide derived from the DYNLL-binding domain of Bim also led to the degradation of Bim as well as of its preferred binding partner Mcl-1. Thus DYNLL regulates the mitochondrial pathway of apoptosis by determining the stability of Bmf, Bim, and Mcl-1 proteins.
中文翻译:

动力蛋白轻链结合决定了 Bcl-2 家族成员 Bmf 和 Bim 的复合物形成和翻译后稳定性。
BH3-only 类 Bcl-2 家族蛋白触发线粒体凋亡。几种机制用于抑制这些蛋白质的促凋亡活性。已提出动力蛋白轻链 (DYNLL) 1 和 2 分别负调节 Bim 和 Bmf 的活性,并且 Bim-DYNLL1 相互作用导致在线粒体上形成大的蛋白质复合物。在这里我们发现 Bim 和 Bmf 与 DYNLL 的两种同工型(DYNLL1 和 DYNLL2)相互作用。DYNLL1/2 不仅诱导 Bim 和 Bmf 的同二聚化,而且导致在无细胞和细胞系统中形成三元复合物 (Bim-DYNLL-Bmf)。DYNLL 诱导的寡聚化稳定了培养细胞中的 Bmf,并抑制了无细胞系统中不依赖泛素的 20S 蛋白酶体的降解。出奇,野生型 Bmf 而非 DYNLL 结合缺陷突变体的过表达诱导不同细胞系中内源性 Bim 的降解,但两种变体都对细胞凋亡敏感。不能与抗凋亡 Bcl-2 蛋白相互作用和诱导细胞凋亡的突变 Bmf 仍然导致 Bim 降解。这些结果表明 Bmf 过表达诱导的 Bim 降解不是由于 Bim 从抗凋亡 Bcl-2 蛋白中的置换,而是 Bim-DYNLL 关联调节的直接结果。源自 Bim 的 DYNLL 结合域的肽也导致 Bim 及其首选结合伙伴 Mcl-1 的降解。因此,DYNLL 通过确定 Bmf、Bim 和 Mcl-1 蛋白的稳定性来调节细胞凋亡的线粒体途径。
更新日期:2019-06-12
中文翻译:

动力蛋白轻链结合决定了 Bcl-2 家族成员 Bmf 和 Bim 的复合物形成和翻译后稳定性。
BH3-only 类 Bcl-2 家族蛋白触发线粒体凋亡。几种机制用于抑制这些蛋白质的促凋亡活性。已提出动力蛋白轻链 (DYNLL) 1 和 2 分别负调节 Bim 和 Bmf 的活性,并且 Bim-DYNLL1 相互作用导致在线粒体上形成大的蛋白质复合物。在这里我们发现 Bim 和 Bmf 与 DYNLL 的两种同工型(DYNLL1 和 DYNLL2)相互作用。DYNLL1/2 不仅诱导 Bim 和 Bmf 的同二聚化,而且导致在无细胞和细胞系统中形成三元复合物 (Bim-DYNLL-Bmf)。DYNLL 诱导的寡聚化稳定了培养细胞中的 Bmf,并抑制了无细胞系统中不依赖泛素的 20S 蛋白酶体的降解。出奇,野生型 Bmf 而非 DYNLL 结合缺陷突变体的过表达诱导不同细胞系中内源性 Bim 的降解,但两种变体都对细胞凋亡敏感。不能与抗凋亡 Bcl-2 蛋白相互作用和诱导细胞凋亡的突变 Bmf 仍然导致 Bim 降解。这些结果表明 Bmf 过表达诱导的 Bim 降解不是由于 Bim 从抗凋亡 Bcl-2 蛋白中的置换,而是 Bim-DYNLL 关联调节的直接结果。源自 Bim 的 DYNLL 结合域的肽也导致 Bim 及其首选结合伙伴 Mcl-1 的降解。因此,DYNLL 通过确定 Bmf、Bim 和 Mcl-1 蛋白的稳定性来调节细胞凋亡的线粒体途径。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号