当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ROS-sensitive biomimetic nanocarriers modulate tumor hypoxia for synergistic photodynamic chemotherapy.
Biomaterials Science ( IF 5.8 ) Pub Date : 2019-08-20 , DOI: 10.1039/c9bm00634f Hang Liu 1 , Wei Jiang , Qin Wang , Lifeng Hang , Yucai Wang , Yanmei Wang
Biomaterials Science ( IF 5.8 ) Pub Date : 2019-08-20 , DOI: 10.1039/c9bm00634f Hang Liu 1 , Wei Jiang , Qin Wang , Lifeng Hang , Yucai Wang , Yanmei Wang
Affiliation
|
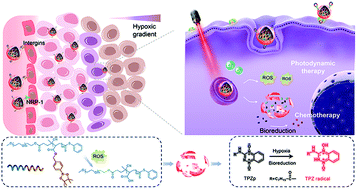
Tumor hypoxia, which is indispensable to tumor propagation and therapy resistance, has been one of the most important factors influencing clinical outcomes. To modulate the hypoxia microenvironment, we herein developed reactive oxygen species (ROS)-sensitive arylboronic ester-based biomimetic nanocarriers co-encapsulated with a photosensitizer chlorin e6 (Ce6) and a hypoxia-activated prodrug tirapazamine (TPZp) for tumor-specific release and synergistic photodynamic chemotherapy. In order to bypass macrophage uptake and improve tumor penetration, the nanocarriers were further modified with the red blood cell membrane and iRGD peptide (denoted as NPs@i-RBMCe6+TPZp). After administration, NPs@i-RBMCe6+TPZp exhibited prolonged blood circulation, selective tumor accumulation and excellent penetration into the tumor interior. Upon light irradiation, ROS were generated by Ce6 for photodynamic therapy (PDT), which subsequently caused dissociation of the ROS-responsive nanocarriers. An enhanced therapeutic effect was further achieved through the activation of TPZp in the aggravated local hypoxia microenvironment. The synergistic cancer therapy based on NPs@i-RBMCe6+TPZp significantly suppressed tumor growth with negligible side effects. The biomimetic nanocarriers have great potential to overcome hypoxia-limited PDT, and significantly improve the anticancer efficacy by synergistic tumor-targeted PDT and hypoxia-activated chemotherapy.
中文翻译:

ROS敏感的仿生纳米载体可调节肿瘤缺氧,以实现协同光动力化学疗法。
肿瘤缺氧是肿瘤扩散和治疗抗性必不可少的,已成为影响临床结果的最重要因素之一。为了调节缺氧的微环境,我们在本文中开发了活性氧(ROS)敏感的基于芳基硼酸酯的仿生纳米载体,与光敏剂二氢卟酚e6(Ce6)和缺氧激活的前药替拉帕明(TPZp)一起包封。协同光动力化学疗法。为了绕过巨噬细胞摄取并改善肿瘤渗透,用红细胞膜和iRGD肽(表示为NPs @ i-RBMCe6 + TPZp)进一步修饰了纳米载体。给药后,NPs @ i-RBMCe6 + TPZp表现出延长的血液循环,选择性的肿瘤积累和极好的向肿瘤内部的渗透。在光照射下 Ce6产生的ROS用于光动力疗法(PDT),随后引起ROS响应性纳米载体解离。通过在严重的局部缺氧微环境中激活TPZp,可以进一步提高治疗效果。基于NPs @ i-RBMCe6 + TPZp的协同癌症治疗显着抑制了肿瘤的生长,且副作用可忽略不计。仿生纳米载体具有克服缺氧受限PDT的巨大潜力,并通过协同靶向肿瘤的PDT和缺氧激活的化学疗法显着提高抗癌功效。基于NPs @ i-RBMCe6 + TPZp的协同癌症治疗显着抑制了肿瘤的生长,且副作用可忽略不计。仿生纳米载体具有克服缺氧受限PDT的巨大潜力,并通过协同靶向肿瘤的PDT和缺氧激活的化学疗法显着提高抗癌功效。基于NPs @ i-RBMCe6 + TPZp的协同癌症治疗显着抑制了肿瘤的生长,且副作用可忽略不计。仿生纳米载体具有克服缺氧受限PDT的巨大潜力,并通过协同靶向肿瘤的PDT和缺氧激活的化学疗法显着提高抗癌功效。
更新日期:2019-06-12
中文翻译:

ROS敏感的仿生纳米载体可调节肿瘤缺氧,以实现协同光动力化学疗法。
肿瘤缺氧是肿瘤扩散和治疗抗性必不可少的,已成为影响临床结果的最重要因素之一。为了调节缺氧的微环境,我们在本文中开发了活性氧(ROS)敏感的基于芳基硼酸酯的仿生纳米载体,与光敏剂二氢卟酚e6(Ce6)和缺氧激活的前药替拉帕明(TPZp)一起包封。协同光动力化学疗法。为了绕过巨噬细胞摄取并改善肿瘤渗透,用红细胞膜和iRGD肽(表示为NPs @ i-RBMCe6 + TPZp)进一步修饰了纳米载体。给药后,NPs @ i-RBMCe6 + TPZp表现出延长的血液循环,选择性的肿瘤积累和极好的向肿瘤内部的渗透。在光照射下 Ce6产生的ROS用于光动力疗法(PDT),随后引起ROS响应性纳米载体解离。通过在严重的局部缺氧微环境中激活TPZp,可以进一步提高治疗效果。基于NPs @ i-RBMCe6 + TPZp的协同癌症治疗显着抑制了肿瘤的生长,且副作用可忽略不计。仿生纳米载体具有克服缺氧受限PDT的巨大潜力,并通过协同靶向肿瘤的PDT和缺氧激活的化学疗法显着提高抗癌功效。基于NPs @ i-RBMCe6 + TPZp的协同癌症治疗显着抑制了肿瘤的生长,且副作用可忽略不计。仿生纳米载体具有克服缺氧受限PDT的巨大潜力,并通过协同靶向肿瘤的PDT和缺氧激活的化学疗法显着提高抗癌功效。基于NPs @ i-RBMCe6 + TPZp的协同癌症治疗显着抑制了肿瘤的生长,且副作用可忽略不计。仿生纳米载体具有克服缺氧受限PDT的巨大潜力,并通过协同靶向肿瘤的PDT和缺氧激活的化学疗法显着提高抗癌功效。































 京公网安备 11010802027423号
京公网安备 11010802027423号