当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of a Competitive Solvent on Binding Enthalpy and Chain Intermixing in Hydrogen-Bonded Layer-by-Layer Films
Macromolecules ( IF 5.1 ) Pub Date : 2019-06-11 00:00:00 , DOI: 10.1021/acs.macromol.9b00650 Victor Selin 1 , Aliaksei Aliakseyeu 1 , John F. Ankner 2 , Svetlana A. Sukhishvili 1
Macromolecules ( IF 5.1 ) Pub Date : 2019-06-11 00:00:00 , DOI: 10.1021/acs.macromol.9b00650 Victor Selin 1 , Aliaksei Aliakseyeu 1 , John F. Ankner 2 , Svetlana A. Sukhishvili 1
Affiliation
|
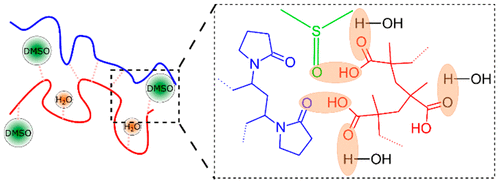
This work is focused on the effect of a small-molecule/hydrogen-bonding competitor on the heat of complexation of hydrogen-bonding polymers in aqueous solutions, as well as on deposition of these molecules within layer-by-layer (LbL) films. Specifically, binding between poly(methacrylic acid) (PMAA) and poly(vinylpyrrolidone) (PVP) under acidic conditions in the presence of dimethyl sulfoxide (DMSO) has been explored. Isothermal titration calorimetry showed that with increasing concentration of DMSO in aqueous solution, the positive enthalpy of formation of PMAA/PVP complexes decreased and switched sign at ∼25 vol % DMSO. This change is interpreted as a transition from endothermal association between PMAA and PVP in water, driven by release of solvation water molecules, to exothermic association driven by the enthalpy of interpolymer hydrogen bonding. This switch is interpreted as destruction of polymer hydration shells by DMSO molecules. The content of DMSO also strongly impacted the growth of LbL films. With an increase in DMSO concentration from 0 to 60 vol %, polymer mass deposited within the bilayer increased, while larger DMSO concentrations suppressed film deposition. The effect of DMSO was also revealed in neutron reflectometry measurements, which showed enhanced interpenetration of deuterated PMAA chains within a hydrogenated LbL matrix at increasing concentration of DMSO. The dependence of film intermixing on DMSO content was strongly history dependent. While assembly of LbL films in aqueous solutions followed by exposure to a mixed solvent post-assembly did not significantly influence the internal film structure, the effect of DMSO on film intermixing was strongest when DMSO was present in the film deposition solutions.
中文翻译:

竞争性溶剂对氢键层合膜中结合焓和链混合的影响
这项工作的重点是小分子/氢键竞争者对水溶液中氢键聚合物的络合热的影响,以及这些分子在逐层(LbL)膜中的沉积。具体地,已经探索了在酸性条件下在二甲基亚砜(DMSO)存在下聚(甲基丙烯酸)(PMAA)和聚(乙烯基吡咯烷酮)(PVP)之间的结合。等温滴定量热法显示,随着水溶液中DMSO浓度的增加,PMAA / PVP复合物形成的正焓降低,并且在约25 vol%DMSO时切换符号。这种变化被解释为由溶剂中水分子释放导致PMAA和PVP在水中从吸热缔合转变而来,互聚物氢键的焓驱动放热缔合。这种转换被解释为DMSO分子破坏了聚合物水合壳。DMSO的含量也强烈影响了LbL膜的生长。随着DMSO浓度从0到60 vol%的增加,沉积在双层中的聚合物质量增加,而较大的DMSO浓度抑制了膜的沉积。DMSO的作用还通过中子反射测量法得到了揭示,该测量表明,随着DMSO浓度的增加,氘化的PMAA链在氢化LbL基质中的互穿性增强。薄膜混合对DMSO含量的依赖性与历史密切相关。
更新日期:2019-06-11
中文翻译:

竞争性溶剂对氢键层合膜中结合焓和链混合的影响
这项工作的重点是小分子/氢键竞争者对水溶液中氢键聚合物的络合热的影响,以及这些分子在逐层(LbL)膜中的沉积。具体地,已经探索了在酸性条件下在二甲基亚砜(DMSO)存在下聚(甲基丙烯酸)(PMAA)和聚(乙烯基吡咯烷酮)(PVP)之间的结合。等温滴定量热法显示,随着水溶液中DMSO浓度的增加,PMAA / PVP复合物形成的正焓降低,并且在约25 vol%DMSO时切换符号。这种变化被解释为由溶剂中水分子释放导致PMAA和PVP在水中从吸热缔合转变而来,互聚物氢键的焓驱动放热缔合。这种转换被解释为DMSO分子破坏了聚合物水合壳。DMSO的含量也强烈影响了LbL膜的生长。随着DMSO浓度从0到60 vol%的增加,沉积在双层中的聚合物质量增加,而较大的DMSO浓度抑制了膜的沉积。DMSO的作用还通过中子反射测量法得到了揭示,该测量表明,随着DMSO浓度的增加,氘化的PMAA链在氢化LbL基质中的互穿性增强。薄膜混合对DMSO含量的依赖性与历史密切相关。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号