当前位置:
X-MOL 学术
›
Microchim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe/C magnetic nanocubes with enhanced peroxidase mimetic activity for colorimetric determination of hydrogen peroxide and glucose
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-06-11 , DOI: 10.1007/s00604-019-3527-1 Fencheng Yang , Guodong Jiang , Feng Yan , Qing Chang
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-06-11 , DOI: 10.1007/s00604-019-3527-1 Fencheng Yang , Guodong Jiang , Feng Yan , Qing Chang
|
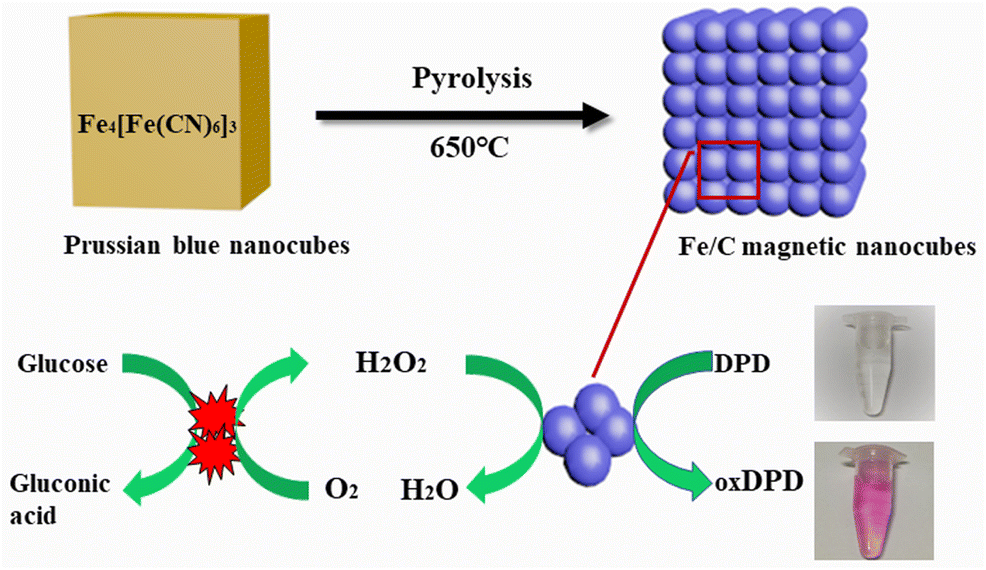
AbstractIron-carbon (Fe/C) magnetic nanocubes were synthesized by direct pyrolysis of Prussian blue nanocubes under an inert gas atmosphere. They are shown to possess intrinsic peroxidase mimicking activity to catalyze the oxidation of peroxidase substrate N,N-diethyl-p-phenylenediamine sulfate salt to form a purple colored product in the presence of H2O2. The values for Km and Vmax are 74 μM and 46 nmol s−1, respectively. Steady-state kinetic analysis also indicates that the catalysis reaction follows a ping-pong mechanism. Based on these findings, an ultrasensitive colorimetric H2O2 assay was worked. Absorbance (best value measured at 550 nm) increases linearly in the 10 nM to 0.2 mM H2O2 concentration range, and the limit of detection is 1.5 nM. The method was also applied to the quantification of glucose, which is oxidized by glucose oxidase in the coexistence of H2O2. The response covers the 0.1 to 500 μM glucose concentration range, and the limit of detection is 16 nM. The method was applied to the determination of H2O2 in rainwater samples. The glucose assay was used to analyze serum samples, and satisfactory results were obtained. Other attractive features include good chemical activity, low cost, easy storage, and high catalytic efficiency. Graphical abstractSchematic presentation of converting Prussian blue nanoparticles into Fe/C magnetic nanocubes by a pyrolysis technique and the use of glucose oxidase and Fe/C magnetic nanocubes to establish a one-step spectrophotometric method for the determination of glucose and hydrogen peroxide.
中文翻译:

Fe/C磁性纳米立方体具有增强的过氧化物酶模拟活性,用于比色测定过氧化氢和葡萄糖
摘要 通过普鲁士蓝纳米立方体在惰性气体气氛下直接热解合成铁碳(Fe/C)磁性纳米立方体。它们被证明具有内在的过氧化物酶模拟活性,可在 H2O2 存在下催化过氧化物酶底物 N,N-二乙基-对苯二胺硫酸盐氧化形成紫色产物。Km 和 Vmax 的值分别为 74 μM 和 46 nmol s-1。稳态动力学分析还表明催化反应遵循乒乓机制。基于这些发现,进行了超灵敏比色 H2O2 测定。吸光度(在 550 nm 处测量的最佳值)在 10 nM 至 0.2 mM H2O2 浓度范围内线性增加,检测限为 1.5 nM。该方法也适用于葡萄糖的定量,在 H2O2 共存下被葡萄糖氧化酶氧化。响应涵盖 0.1 至 500 μM 葡萄糖浓度范围,检测限为 16 nM。该方法适用于雨水样品中H2O2的测定。用葡萄糖测定法分析血清样品,得到满意的结果。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。检测限为 16 nM。该方法适用于雨水样品中H2O2的测定。用葡萄糖测定法分析血清样品,得到满意的结果。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。检测限为 16 nM。该方法适用于雨水样品中H2O2的测定。用葡萄糖测定法分析血清样品,得到满意的结果。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。
更新日期:2019-06-11
中文翻译:

Fe/C磁性纳米立方体具有增强的过氧化物酶模拟活性,用于比色测定过氧化氢和葡萄糖
摘要 通过普鲁士蓝纳米立方体在惰性气体气氛下直接热解合成铁碳(Fe/C)磁性纳米立方体。它们被证明具有内在的过氧化物酶模拟活性,可在 H2O2 存在下催化过氧化物酶底物 N,N-二乙基-对苯二胺硫酸盐氧化形成紫色产物。Km 和 Vmax 的值分别为 74 μM 和 46 nmol s-1。稳态动力学分析还表明催化反应遵循乒乓机制。基于这些发现,进行了超灵敏比色 H2O2 测定。吸光度(在 550 nm 处测量的最佳值)在 10 nM 至 0.2 mM H2O2 浓度范围内线性增加,检测限为 1.5 nM。该方法也适用于葡萄糖的定量,在 H2O2 共存下被葡萄糖氧化酶氧化。响应涵盖 0.1 至 500 μM 葡萄糖浓度范围,检测限为 16 nM。该方法适用于雨水样品中H2O2的测定。用葡萄糖测定法分析血清样品,得到满意的结果。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。检测限为 16 nM。该方法适用于雨水样品中H2O2的测定。用葡萄糖测定法分析血清样品,得到满意的结果。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。检测限为 16 nM。该方法适用于雨水样品中H2O2的测定。用葡萄糖测定法分析血清样品,得到满意的结果。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。其他吸引人的特点包括良好的化学活性、低成本、易于储存和高催化效率。图形摘要通过热解技术将普鲁士蓝纳米颗粒转化为 Fe/C 磁性纳米立方体的示意图,并使用葡萄糖氧化酶和 Fe/C 磁性纳米立方体建立了一种测定葡萄糖和过氧化氢的一步分光光度法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号