当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carboxylate-Substituted Polythiophenes for Efficient Fullerene-Free Polymer Solar Cells: The Effect of Chlorination on Their Properties
Macromolecules ( IF 5.1 ) Pub Date : 2019-06-11 00:00:00 , DOI: 10.1021/acs.macromol.9b00793 Qi Wang 1 , Miaomiao Li 1, 2, 3 , Xiaowei Zhang 1 , Yunpeng Qin 4 , Junke Wang 3 , Jidong Zhang 5 , Jianhui Hou 4 , René A. J. Janssen 3 , Yanhou Geng 1, 2
Macromolecules ( IF 5.1 ) Pub Date : 2019-06-11 00:00:00 , DOI: 10.1021/acs.macromol.9b00793 Qi Wang 1 , Miaomiao Li 1, 2, 3 , Xiaowei Zhang 1 , Yunpeng Qin 4 , Junke Wang 3 , Jidong Zhang 5 , Jianhui Hou 4 , René A. J. Janssen 3 , Yanhou Geng 1, 2
Affiliation
|
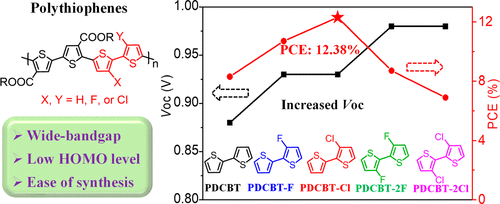
Two new wide-bandgap polythiophenes, i.e., poly[5,5′-bis(2-hexyldecyl)-(2,2′-bithiophene)-4,4′-dicarboxylate-alt-5,5′-3-chloro-2,2′-bithiophene] (PDCBT-Cl) and poly[5,5′-bis(2-hexyldecyl)-(2,2′-bithiophene)-4,4′-dicarboxylate-alt-5,5′-3,3′-dichloro-2,2′-bithiophene] (PDCBT-2Cl) comprising 3-chloro-2,2′-bithiophene and 3,3′-dichloro-2,2′-bithiophene moieties, respectively, were synthesized for fullerene-free polymer solar cells (PSCs). For comparison, three other polymers based on [2,2′-bithiophene]-4,4′-dicarboxylate (DCBT), i.e., PDCBT, PDCBT-F, and PDCBT-2F with 2,2′-bithiophene, 3-fluoro-2,2′-bithiophene, and 3,3′-difluoro-2,2′-bithiophene as comonomers, respectively, were also prepared. PSC devices were fabricated with these polymers as donor materials and ITIC-Th1 as acceptor. The incorporation of chlorine (Cl) or fluorine (F) atoms into polymers both efficiently downshifted the highest occupied molecular orbital (HOMO) energy levels, leading to higher open-circuit voltage (Voc) in the PSCs. Owing to the proper phase-separated morphology with favorable molecular packing and miscibility, the device based on PDCBT-Cl:ITIC-Th1 exhibited efficient exciton dissociation and charge collection as well as weak charge recombination and thereby displayed the best power conversion efficiency (PCE) up to 12.38%. The devices based on other polymers showed inferior PCEs (8.14% for PDCBT, 10.85% for PDCBT-F, 8.48% for PDCBT-2F, and 6.94% for PDCBT-2Cl). The monomers that are used to make PDCBT-Cl can be synthesized in relatively large scale from commercial available chemicals. All these indicate that PDCBT-Cl is a promising donor material for the large area fabrication of high-performance fullerene-free PSCs.
中文翻译:

高效无富勒烯聚合物太阳能电池的羧酸取代聚噻吩:氯化对其性能的影响
两个新的宽带隙聚噻吩,即,聚[5,5'-双(2-己基癸) - (2,2'-联噻吩)-4,4'-二羧酸酯ALT -5,5'- -3-氯2,2'-联噻吩](PDCBT-C1)和聚[5,5'-双(2-己基癸) - (2,2'-联噻吩)-4,4'-二羧酸酯中高音-5,5'-3,3'-dichloro-2,2'-bithiophene](PDCBT-2Cl)包含3-chloro-2,2'-bithiophene和3,3'-dichloro-2,2'-bithiophene分别合成了不含富勒烯的聚合物太阳能电池(PSC)的部分。为了比较,基于[2,2'-联噻吩] -4,4'-二羧酸酯(DCBT)的其他三种聚合物,即PDCBT,PDCBT-F和PDCBT-2F与2,2'-联噻吩,3-氟还分别制备了作为共聚单体的-2,2′-联噻吩和3,3′-二氟-2,2′-联噻吩。使用这些聚合物作为供体材料并使用ITIC-Th1作为受体来制造PSC器件。将氯(Cl)或氟(F)原子结合到聚合物中均有效地降低了最高占据分子轨道(HOMO)的能级,从而导致更高的开路电压(V oc)。由于具有良好的分子堆积和可混溶性的适当的相分离形态,基于PDCBT-Cl:ITIC-Th1的器件显示出有效的激子离解和电荷收集以及弱的电荷重组,因此显示出最佳的功率转换效率(PCE)高达12.38%。基于其他聚合物的设备显示出较差的PCE(PDCBT为8.14%,PDCBT-F为10.85%,PDCBT-2F为8.48%,PDCBT-2Cl为6.94%)。用于制备PDCBT-Cl的单体可以从市售化学品中以相对较大的比例合成。所有这些表明,PDCBT-Cl是大面积制造高性能无富勒烯PSC的有前途的供体材料。
更新日期:2019-06-11
中文翻译:

高效无富勒烯聚合物太阳能电池的羧酸取代聚噻吩:氯化对其性能的影响
两个新的宽带隙聚噻吩,即,聚[5,5'-双(2-己基癸) - (2,2'-联噻吩)-4,4'-二羧酸酯ALT -5,5'- -3-氯2,2'-联噻吩](PDCBT-C1)和聚[5,5'-双(2-己基癸) - (2,2'-联噻吩)-4,4'-二羧酸酯中高音-5,5'-3,3'-dichloro-2,2'-bithiophene](PDCBT-2Cl)包含3-chloro-2,2'-bithiophene和3,3'-dichloro-2,2'-bithiophene分别合成了不含富勒烯的聚合物太阳能电池(PSC)的部分。为了比较,基于[2,2'-联噻吩] -4,4'-二羧酸酯(DCBT)的其他三种聚合物,即PDCBT,PDCBT-F和PDCBT-2F与2,2'-联噻吩,3-氟还分别制备了作为共聚单体的-2,2′-联噻吩和3,3′-二氟-2,2′-联噻吩。使用这些聚合物作为供体材料并使用ITIC-Th1作为受体来制造PSC器件。将氯(Cl)或氟(F)原子结合到聚合物中均有效地降低了最高占据分子轨道(HOMO)的能级,从而导致更高的开路电压(V oc)。由于具有良好的分子堆积和可混溶性的适当的相分离形态,基于PDCBT-Cl:ITIC-Th1的器件显示出有效的激子离解和电荷收集以及弱的电荷重组,因此显示出最佳的功率转换效率(PCE)高达12.38%。基于其他聚合物的设备显示出较差的PCE(PDCBT为8.14%,PDCBT-F为10.85%,PDCBT-2F为8.48%,PDCBT-2Cl为6.94%)。用于制备PDCBT-Cl的单体可以从市售化学品中以相对较大的比例合成。所有这些表明,PDCBT-Cl是大面积制造高性能无富勒烯PSC的有前途的供体材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号