Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2019-06-08 , DOI: 10.1016/j.cej.2019.06.022 Guilin He , Tuqiao Zhang , Feifei Zheng , Cong Li , Qingzhou Zhang , Feilong Dong , Yuan Huang
|
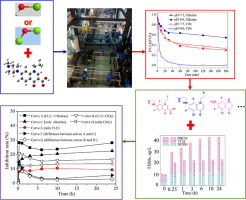
Fleroxacin (FLE) is an emerging third-generation fluoroquinolone antibacterial agent (FQs) that has been frequently detected in aqueous environments. However, there is a lack of sufficient knowledge regarding the transformation mechanisms of FLE within drinking water distribution systems (DWDSs) when residual chlorine and ClO2 are present. To address this gap, this study makes the first attempt to explore the kinetics, transformation byproducts and toxicity variations during fleroxacin (FLE, an emergent pollutant) degradation by chlorine and ClO2 in a pilot-scale DWDSs. The obtained results show that (i) the FLE degradation rate by chlorine was higher than by ClO2 at pH 7.4 in the pilot-scale DWDSs; (ii) the degradation efficiency of FLE was significantly affected by pH, with FLE degradation by chlorine possessing the highest rate at neutral pH, and the degradation rate was positively correlated with pH (from 6.5 to 9) during the ClO2 disinfection process; (iii) pipe materials can appreciably affect the relative performance of the FLE degradation efficiency by chlorine and ClO2; (iv) seven and eight intermediates are identified during chlorination and ClO2 oxidation, respectively, and the cleavage of the piperazine group was the committed step and the main oxidation reaction, and (v) the toxicity assessment demonstrates that the toxicity of FLE chlorination and ClO2 are both higher than the blank experiment, and ClO2 disinfection can reduce the potential risk compared to the chlorine disinfection.
中文翻译:

饮用水分配系统中氟沙星与氯和二氧化氯的反应:动力学,转化机理和毒性评估
氟罗沙星(FLE)是一种新兴的第三代氟喹诺酮类抗菌剂(FQs),已在水性环境中被频繁检测到。但是,当存在残留的氯和ClO 2时,关于饮用水分配系统(DWDS)中FLE的转化机理缺乏足够的知识。为了解决这一差距,本研究首次尝试探索了中试DWDS中氯和ClO 2降解氟沙星(FLE,一种新兴污染物)过程中的动力学,转化副产物和毒性变化。获得的结果表明(i)氯对FLE的降解率高于ClO 2对FLE的降解率在中试规模DWDS的pH值为7.4的情况下;(ii)pH值对FLE的降解效率有显着影响,在中性pH值下氯气对FLE的降解率最高,并且在ClO 2消毒过程中降解率与pH呈正相关(从6.5到9);(iii)管道材料会明显影响氯和ClO 2对FLE降解效率的相对性能;(iv)在氯化和ClO 2氧化过程中分别鉴定出7种和8种中间体,哌嗪基团的裂解是主要步骤和主要的氧化反应,并且(v)毒性评估表明FLE氯化的毒性和CLO 2两者均高于空白实验,并且与氯气消毒相比,ClO 2消毒可降低潜在风险。






























 京公网安备 11010802027423号
京公网安备 11010802027423号