当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PtII‐Catalyzed Hydroxylation of Terminal Aliphatic C(sp3)−H Bonds with Molecular Oxygen
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-07-09 , DOI: 10.1002/chem.201901803 Michiel Janssen 1 , Dirk E. De Vos 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-07-09 , DOI: 10.1002/chem.201901803 Michiel Janssen 1 , Dirk E. De Vos 1
Affiliation
|
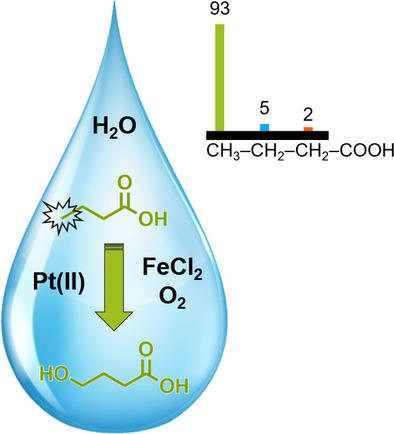
The practical application of Shilov‐type Pt catalysis to the selective hydroxylation of terminal aliphatic C−H bonds remains a formidable challenge, due to difficulties in replacing PtIV with a more economically viable oxidant, particularly O2. We report the potential of employing FeCl2 as a suitable redox mediator to overcome the kinetic hurdles related to the direct use of O2 in the Pt reoxidation. For the selective conversion of butyric acid to γ‐hydroxybutyric acid (GHB), a significantly enhanced catalyst activity and stability (turnover numbers (TON)>30) were achieved under 20 bar O2 in comparison to current state‐of‐the‐art systems (TON<10). In this regard, essential reaction parameters affecting the overall activity were identified, along with specific additives to attain catalyst stability at longer reaction times. Notably, deactivation by reduction to Pt0 was prevented by the addition of monodentate pyridine derivatives, such as 2‐fluoropyridine, but also by introducing varying partial pressures of N2 in the gaseous atmosphere. Finally, stability tests revealed the involvement of PtII and FeCl2 in catalyzing the non‐selective overoxidation of GHB. Accordingly, in situ esterification with boric acid proved to be a suitable strategy to maintain enhanced selectivities at much higher conversions (TON>60). Altogether, a useful catalytic system for the selective hydroxylation of primary aliphatic C−H bonds with O2 is presented.
中文翻译:

PtII催化的末端脂肪C(sp3)-H键与分子氧的羟基化作用
由于难以用更经济可行的氧化剂(尤其是O 2)取代Pt IV,Shilov型Pt催化在末端脂肪族CH键选择性羟基化方面的实际应用仍然是一个艰巨的挑战。我们报告了使用FeCl 2作为合适的氧化还原介体来克服与Pt再氧化中直接使用O 2有关的动力学障碍的潜力。为了将丁酸选择性转化为γ-羟基丁酸(GHB),在20 bar O 2下获得了显着增强的催化剂活性和稳定性(周转数(TON)> 30)。与当前最先进的系统(TON <10)相比。在这方面,确定了影响整体活性的基本反应参数,以及用于在更长的反应时间获得催化剂稳定性的特定添加剂。值得注意的是,通过添加单齿吡啶衍生物(例如2-氟吡啶),以及通过在气态气氛中引入变化的N 2分压,可以防止还原为Pt 0引起的失活。最后,稳定性测试表明Pt II和FeCl 2参与其中催化GHB的非选择性过氧化。因此,用硼酸原位酯化证明是在更高的转化率(TON> 60)下保持提高的选择性的合适策略。总的来说,提出了一种有用的催化体系,用于用O 2选择性地脂族脂肪族碳氢键的选择性羟基化。
更新日期:2019-07-09
中文翻译:

PtII催化的末端脂肪C(sp3)-H键与分子氧的羟基化作用
由于难以用更经济可行的氧化剂(尤其是O 2)取代Pt IV,Shilov型Pt催化在末端脂肪族CH键选择性羟基化方面的实际应用仍然是一个艰巨的挑战。我们报告了使用FeCl 2作为合适的氧化还原介体来克服与Pt再氧化中直接使用O 2有关的动力学障碍的潜力。为了将丁酸选择性转化为γ-羟基丁酸(GHB),在20 bar O 2下获得了显着增强的催化剂活性和稳定性(周转数(TON)> 30)。与当前最先进的系统(TON <10)相比。在这方面,确定了影响整体活性的基本反应参数,以及用于在更长的反应时间获得催化剂稳定性的特定添加剂。值得注意的是,通过添加单齿吡啶衍生物(例如2-氟吡啶),以及通过在气态气氛中引入变化的N 2分压,可以防止还原为Pt 0引起的失活。最后,稳定性测试表明Pt II和FeCl 2参与其中催化GHB的非选择性过氧化。因此,用硼酸原位酯化证明是在更高的转化率(TON> 60)下保持提高的选择性的合适策略。总的来说,提出了一种有用的催化体系,用于用O 2选择性地脂族脂肪族碳氢键的选择性羟基化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号