当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Some Reactions with Indane‐1,3‐dione: A Facile Synthesis of Pentacycline Heterocyclic Analogues
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-06-06 , DOI: 10.1002/jhet.3572 Fathy M. Abdelrazek 1, 2 , Peter Metz 2 , Anne Jaeger 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-06-06 , DOI: 10.1002/jhet.3572 Fathy M. Abdelrazek 1, 2 , Peter Metz 2 , Anne Jaeger 2
Affiliation
|
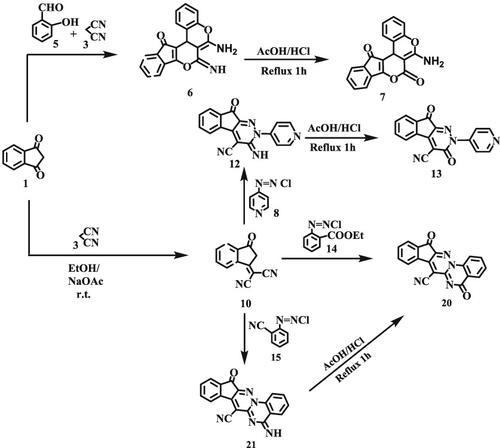
Indane‐1,3‐dione 1 reacts with salicylaldehyde 5 and malononitrile 3 to afford 6‐amino‐7‐imino‐7H‐indeno‐[2′,1′:5,6]‐pyrano‐[3,4‐c]‐chromene 6, which could be transformed into the corresponding 7‐oxo derivative 7. 2‐(3‐Oxoindan‐1‐ylidene)‐malononitrile 10 couples with the diazonium salts 8, 14, and 15 to afford after cyclization the indeno‐[2,1‐c]‐pyridazine 13 and the indeno‐[2′,1′:3,4]‐pyridazino‐[1,6‐a]‐quinazoline derivatives 20 and 21, respectively.
中文翻译:

与茚满-1,3-二酮的某些反应:五环素杂环类似物的简便合成
茚满1,3-二酮1与水杨醛5和丙二腈3反应生成6-氨基-7-亚氨基-7 H-茚满[2',1':5,6]-吡喃并[3,4 - c ] -chromene 6,可以将其转化为相应的7-oxo衍生物7。2-(3-氧代茚满-1-亚基) -丙二腈10个耦合与重氮盐8,14,和15环化的茚并后,得到[2,1- c ^ ] -哒嗪13和茚并[2', 1':3,4]-哒嗪-[1,6- a ]-喹唑啉衍生物20和分别为21。
更新日期:2019-06-06
中文翻译:

与茚满-1,3-二酮的某些反应:五环素杂环类似物的简便合成
茚满1,3-二酮1与水杨醛5和丙二腈3反应生成6-氨基-7-亚氨基-7 H-茚满[2',1':5,6]-吡喃并[3,4 - c ] -chromene 6,可以将其转化为相应的7-oxo衍生物7。2-(3-氧代茚满-1-亚基) -丙二腈10个耦合与重氮盐8,14,和15环化的茚并后,得到[2,1- c ^ ] -哒嗪13和茚并[2', 1':3,4]-哒嗪-[1,6- a ]-喹唑啉衍生物20和分别为21。







































 京公网安备 11010802027423号
京公网安备 11010802027423号