当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic basis of Nek7 activation through Nek9 binding and induced dimerization.
Nature Communications ( IF 14.7 ) Pub Date : 2015-Nov-02 , DOI: 10.1038/ncomms9771
Tamanna Haq 1, 2 , Mark W Richards 1, 2 , Selena G Burgess 1, 2 , Pablo Gallego 3 , Sharon Yeoh 1, 2 , Laura O'Regan 1, 2 , David Reverter 3 , Joan Roig 4 , Andrew M Fry 1, 2 , Richard Bayliss 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2015-Nov-02 , DOI: 10.1038/ncomms9771
Tamanna Haq 1, 2 , Mark W Richards 1, 2 , Selena G Burgess 1, 2 , Pablo Gallego 3 , Sharon Yeoh 1, 2 , Laura O'Regan 1, 2 , David Reverter 3 , Joan Roig 4 , Andrew M Fry 1, 2 , Richard Bayliss 1, 2
Affiliation
|
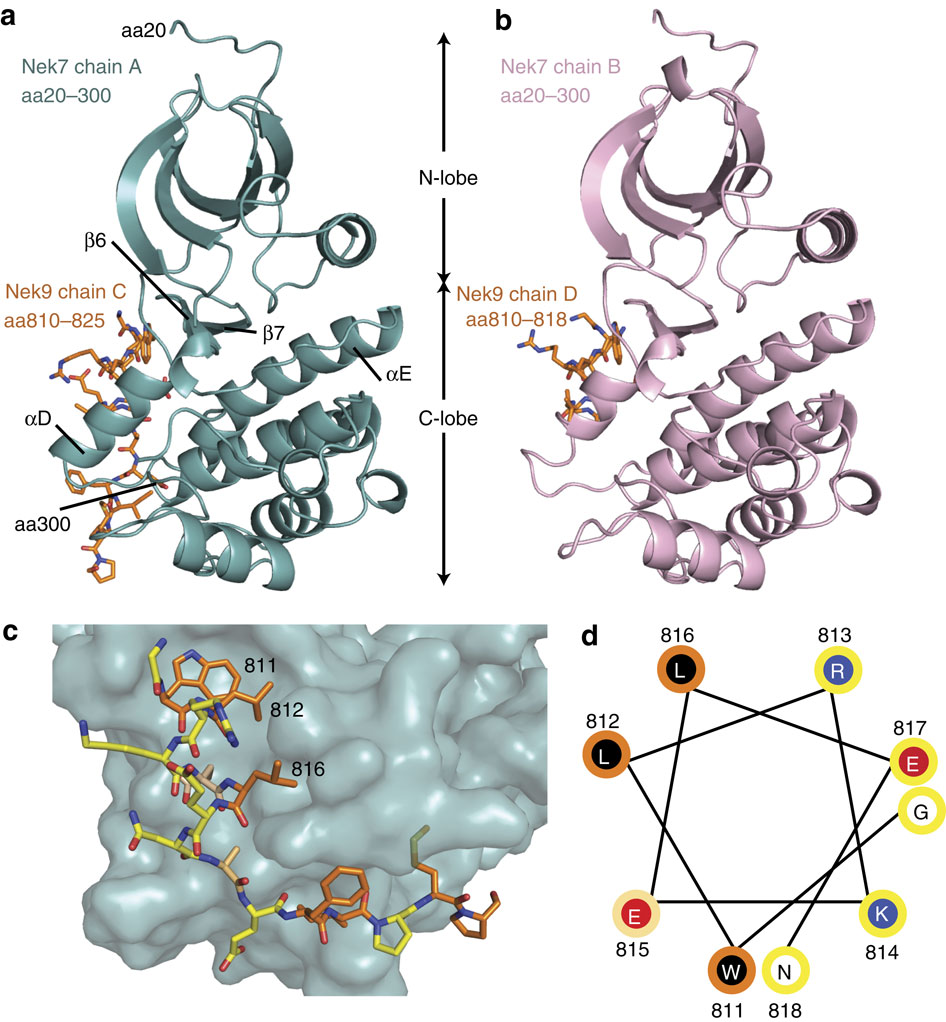
Mitotic spindle assembly requires the regulated activities of protein kinases such as Nek7 and Nek9. Nek7 is autoinhibited by the protrusion of Tyr97 into the active site and activated by the Nek9 non-catalytic C-terminal domain (CTD). CTD binding apparently releases autoinhibition because mutation of Tyr97 to phenylalanine increases Nek7 activity independently of Nek9. Here we find that self-association of the Nek9-CTD is needed for Nek7 activation. We map the minimal Nek7 binding region of Nek9 to residues 810-828. A crystal structure of Nek7(Y97F) bound to Nek9(810-828) reveals a binding site on the C-lobe of the Nek7 kinase domain. Nek7(Y97F) crystallizes as a back-to-back dimer between kinase domain N-lobes, in which the specific contacts within the interface are coupled to the conformation of residue 97. Hence, we propose that the Nek9-CTD activates Nek7 through promoting back-to-back dimerization that releases the autoinhibitory tyrosine residue, a mechanism conserved in unrelated kinase families.
中文翻译:

通过 Nek9 结合和诱导二聚化激活 Nek7 的机制基础。
有丝分裂纺锤体组装需要蛋白激酶如 Nek7 和 Nek9 的调节活性。Nek7 被 Tyr97 突入活性位点自动抑制,并被 Nek9 非催化 C 末端结构域 (CTD) 激活。CTD 结合显然会释放自身抑制,因为 Tyr97 突变为苯丙氨酸会独立于 Nek9 增加 Nek7 的活性。在这里,我们发现 Nek7 激活需要 Nek9-CTD 的自关联。我们将 Nek9 的最小 Nek7 结合区域映射到残基 810-828。与 Nek9(810-828) 结合的 Nek7(Y97F) 晶体结构揭示了 Nek7 激酶结构域 C 瓣上的结合位点。Nek7(Y97F) 在激酶结构域 N-叶之间结晶为背靠背二聚体,其中界面内的特定接触与残基 97 的构象耦合。因此,
更新日期:2015-11-05
中文翻译:

通过 Nek9 结合和诱导二聚化激活 Nek7 的机制基础。
有丝分裂纺锤体组装需要蛋白激酶如 Nek7 和 Nek9 的调节活性。Nek7 被 Tyr97 突入活性位点自动抑制,并被 Nek9 非催化 C 末端结构域 (CTD) 激活。CTD 结合显然会释放自身抑制,因为 Tyr97 突变为苯丙氨酸会独立于 Nek9 增加 Nek7 的活性。在这里,我们发现 Nek7 激活需要 Nek9-CTD 的自关联。我们将 Nek9 的最小 Nek7 结合区域映射到残基 810-828。与 Nek9(810-828) 结合的 Nek7(Y97F) 晶体结构揭示了 Nek7 激酶结构域 C 瓣上的结合位点。Nek7(Y97F) 在激酶结构域 N-叶之间结晶为背靠背二聚体,其中界面内的特定接触与残基 97 的构象耦合。因此,







































 京公网安备 11010802027423号
京公网安备 11010802027423号