当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium Benzenedithiolate Catholytes for Rechargeable Lithium Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-06-07 , DOI: 10.1002/adfm.201902223 Fengli Li 1 , Yubing Si 2 , Bingjie Liu 3 , Zhongjun Li 1 , Yongzhu Fu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-06-07 , DOI: 10.1002/adfm.201902223 Fengli Li 1 , Yubing Si 2 , Bingjie Liu 3 , Zhongjun Li 1 , Yongzhu Fu 1
Affiliation
|
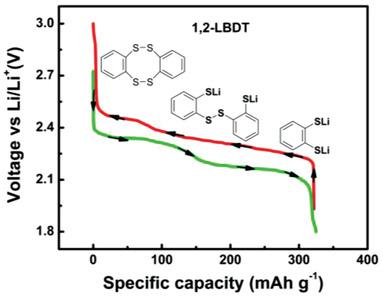
Organic electrode materials have become a vibrant area of research. Lithium benzenedithiolate (LBDT) consists of two –SLi groups that could donate 2Li+ and 2e− in oxidation reactions, thus being a potential high‐capacity organic cathode material for rechargeable lithium batteries. Herein, 1,2‐, 1,3‐, and 1,4‐LBDTs are investigated to elucidate the relationship of their redox chemistry and effect of lithium thiolate position on their electrochemical behavior experimentally and theoretically. High‐performance liquid chromatography in tandem with quadrupole time‐of‐flight mass spectrometry is used, for the first time, to separate and identify the charge and discharge products of these compounds in lithium batteries. During the charging process, 1,2‐, 1,3‐ and 1,4‐LBDTs are mainly converted to the cyclic dimer, trimer, and tetramer respectively. While in the discharge process, the initial lithiated materials are recovered. The cyclic dimer of 1,2‐LBDT shows the lowest discharge overpotential and fast kinetics, which are related to the easiness of the 2nd lithiation process. It delivers an initial specific capacity of 340 mAh g−1 and retains 84.1% of the initial capacity over 100 cycles at C/2 rate. In addition, it shows much better rate capability than the other two LBDTs. The structure–performance relationship of LBDT in lithium batteries is correlated.
中文翻译:

苯二硫代锂锂可充电锂电池的阴极液
有机电极材料已成为一个充满活力的研究领域。锂benzenedithiolate(LBDT)由可能捐赠2LI 2个-SLi组的+和2e -在氧化反应中,因此成为可再充电锂电池的潜在高容量有机阴极材料。在本文中,对1,2-,1,3-和1,4-LBDTs进行了研究,以从实验和理论上阐明它们的氧化还原化学和硫醇锂位置对其电化学行为的影响。高性能液相色谱与四极杆飞行时间质谱联用,首次用于分离和鉴定锂电池中这些化合物的充电和放电产物。在充电过程中,主要将1,2,1,2,3,3,1,4-LBDT分别转化为循环二聚体,三聚体和四聚体。在放电过程中,最初的锂化材料被回收。1的循环二聚体 2-LBDT显示出最低的过电势和快速的动力学特性,这与第二次锂化过程的难易程度有关。它的初始比容量为340 mAh g-1并以C / 2速率在100个循环中保留84.1%的初始容量。此外,它显示出比其他两个LBDT更好的速率能力。锂电池中LBDT的结构与性能之间的关系是相关的。
更新日期:2019-06-07
中文翻译:

苯二硫代锂锂可充电锂电池的阴极液
有机电极材料已成为一个充满活力的研究领域。锂benzenedithiolate(LBDT)由可能捐赠2LI 2个-SLi组的+和2e -在氧化反应中,因此成为可再充电锂电池的潜在高容量有机阴极材料。在本文中,对1,2-,1,3-和1,4-LBDTs进行了研究,以从实验和理论上阐明它们的氧化还原化学和硫醇锂位置对其电化学行为的影响。高性能液相色谱与四极杆飞行时间质谱联用,首次用于分离和鉴定锂电池中这些化合物的充电和放电产物。在充电过程中,主要将1,2,1,2,3,3,1,4-LBDT分别转化为循环二聚体,三聚体和四聚体。在放电过程中,最初的锂化材料被回收。1的循环二聚体 2-LBDT显示出最低的过电势和快速的动力学特性,这与第二次锂化过程的难易程度有关。它的初始比容量为340 mAh g-1并以C / 2速率在100个循环中保留84.1%的初始容量。此外,它显示出比其他两个LBDT更好的速率能力。锂电池中LBDT的结构与性能之间的关系是相关的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号