当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enlarged Interlayer Spacing in Cobalt–Manganese Layered Double Hydroxide Guiding Transformation to Layered Structure for High Supercapacitance
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-06-07 00:00:00 , DOI: 10.1021/acsami.9b05564 Ximeng Liu 1 , Lei Zhang 1 , Xiaorui Gao 1, 2 , Cao Guan 3 , Yating Hu 1 , John Wang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-06-07 00:00:00 , DOI: 10.1021/acsami.9b05564 Ximeng Liu 1 , Lei Zhang 1 , Xiaorui Gao 1, 2 , Cao Guan 3 , Yating Hu 1 , John Wang 1
Affiliation
|
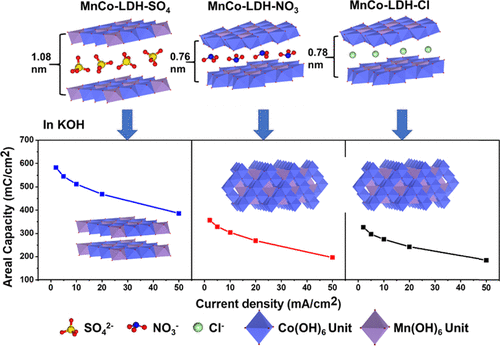
Cobalt–manganese layered double hydroxide (CoMn-LDH) has been known as a highly desired cathode material used with an alkaline electrolyte. However, the layered double hydroxide structure is unstable and changes almost instantly in alkaline solution due to the instability of a manganese(III) ion. Thus, it is important to investigate the true active phase for designing efficient electrode materials. In this work, the metal–organic framework is used as a templating precursor to derive CoMn-LDH from three different manganese solutions, namely, MnSO4, Mn(NO3)2, and MnCl2. Anions in the solutions participate in the derivation process and strongly affect the layer structure, phase transformation process, and charge storage properties of the resulting materials. CoMn-LDH synthesized from manganese sulfate solution exhibits the largest interlayer spacing of 1.08 nm, and more interestingly, the layered structure can well be retained in KOH solution, while the other two synthesized from manganese chloride and nitrate solutions transform into the spinel structure. As a cathode material, it delivers a high areal capacity of 582.07 mC/cm2 at 2 mA/cm2, which is about 100% higher than those of the other two samples. The present work explores the active phase of CoMn-LDH in the alkaline electrolyte and proposes a potential mechanism of the phase transformation, which provides insights into understanding and designing of the active electrode materials for stable and high-performing supercapacitors in an alkaline environment.
中文翻译:

钴-锰层状双氢氧化物中的扩大层间距,指导转变为高超级电容的层状结构
钴锰层状双氢氧化物(CoMn-LDH)被公认为与碱性电解质一起使用的极受欢迎的阴极材料。然而,由于锰(III)离子的不稳定性,层状双氢氧化物结构是不稳定的,并且在碱性溶液中几乎立即发生变化。因此,研究真正的活性相对于设计有效的电极材料很重要。在这项工作中,金属有机骨架被用作模板前体,以从三种不同的锰溶液MnSO 4,Mn(NO 3)2和MnCl 2衍生出CoMn-LDH。。溶液中的阴离子参与衍生过程,并严重影响所得材料的层结构,相变过程和电荷存储特性。由硫酸锰溶液合成的CoMn-LDH表现出最大的层间间距1.08 nm,更有趣的是,层状结构可以很好地保留在KOH溶液中,而由氯化锰和硝酸盐溶液合成的其他两种转化成尖晶石结构。作为阴极材料,它在2 mA / cm 2时可提供582.07 mC / cm 2的高面容量,比其他两个样本的样本高约100%。本工作探讨了碱性电解质中CoMn-LDH的活性相,并提出了一种潜在的相变机理,从而为了解和设计用于碱性环境中稳定和高性能超级电容器的活性电极材料提供了见识。
更新日期:2019-06-07
中文翻译:

钴-锰层状双氢氧化物中的扩大层间距,指导转变为高超级电容的层状结构
钴锰层状双氢氧化物(CoMn-LDH)被公认为与碱性电解质一起使用的极受欢迎的阴极材料。然而,由于锰(III)离子的不稳定性,层状双氢氧化物结构是不稳定的,并且在碱性溶液中几乎立即发生变化。因此,研究真正的活性相对于设计有效的电极材料很重要。在这项工作中,金属有机骨架被用作模板前体,以从三种不同的锰溶液MnSO 4,Mn(NO 3)2和MnCl 2衍生出CoMn-LDH。。溶液中的阴离子参与衍生过程,并严重影响所得材料的层结构,相变过程和电荷存储特性。由硫酸锰溶液合成的CoMn-LDH表现出最大的层间间距1.08 nm,更有趣的是,层状结构可以很好地保留在KOH溶液中,而由氯化锰和硝酸盐溶液合成的其他两种转化成尖晶石结构。作为阴极材料,它在2 mA / cm 2时可提供582.07 mC / cm 2的高面容量,比其他两个样本的样本高约100%。本工作探讨了碱性电解质中CoMn-LDH的活性相,并提出了一种潜在的相变机理,从而为了解和设计用于碱性环境中稳定和高性能超级电容器的活性电极材料提供了见识。































 京公网安备 11010802027423号
京公网安备 11010802027423号