当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor-Specific Silencing of Tissue Factor Suppresses Metastasis and Prevents Cancer-Associated Hypercoagulability
Nano Letters ( IF 9.6 ) Pub Date : 2019-06-07 00:00:00 , DOI: 10.1021/acs.nanolett.9b01785 Shaoli Liu 1, 2 , Yinlong Zhang 1 , Xiao Zhao 1 , Jing Wang 1 , Chunzhi Di 1, 2 , Ying Zhao 1 , Tianjiao Ji 1 , Keman Cheng 1 , Yongwei Wang 1 , Long Chen 1, 2 , Yingqiu Qi 1, 2, 3 , Suping Li 1, 2 , Guangjun Nie 1, 2
Nano Letters ( IF 9.6 ) Pub Date : 2019-06-07 00:00:00 , DOI: 10.1021/acs.nanolett.9b01785 Shaoli Liu 1, 2 , Yinlong Zhang 1 , Xiao Zhao 1 , Jing Wang 1 , Chunzhi Di 1, 2 , Ying Zhao 1 , Tianjiao Ji 1 , Keman Cheng 1 , Yongwei Wang 1 , Long Chen 1, 2 , Yingqiu Qi 1, 2, 3 , Suping Li 1, 2 , Guangjun Nie 1, 2
Affiliation
|
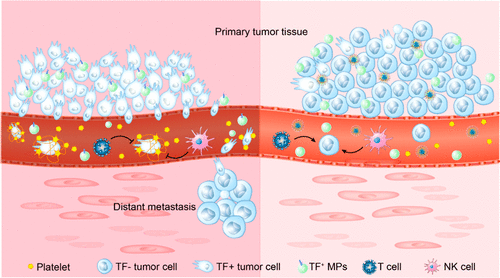
Within tumors, the coagulation-inducing protein tissue factor (TF), a major initiator of blood coagulation, has been shown to play a critical role in the hematogenous metastasis of tumors, due to its effects on tumor hypercoagulability and on the mediation of interactions between platelets and tumor cells. Targeting tumor-associated TF has therefore great therapeutic potential for antimetastasis therapy and preventing thrombotic complication in cancer patients. Herein, we reported a novel peptide-based nanoparticle that targets delivery and release of small interfering RNA (siRNA) into the tumor site to silence the expression of tumor-associated TF. We showed that suppression of TF expression in tumor cells blocks platelet adhesion surrounding tumor cells in vitro. The downregulation of TF expression in intravenously administered tumor cells (i.e., simulated circulating tumor cells [CTCs]) prevented platelet adhesion around CTCs and decreased CTCs survival in the lung. In a breast cancer mouse model, siRNA-containing nanoparticles efficiently attenuated TF expression in the tumor microenvironment and remarkably reduced the amount of lung metastases in both an experimental lung metastasis model and tumor-bearing mice. What’s more, this strategy reversed the hypercoagulable state of the tumor bearing mice by decreasing the generation of thrombin-antithrombin complexes (TAT) and activated platelets, both of which are downstream products of TF. Our study describes a promising approach to combat metastasis and prevent cancer-associated thrombosis, which advances TF as a therapeutic target toward clinic applications.
中文翻译:

组织因子的肿瘤特异性沉默抑制转移并预防癌症相关的高凝性
在肿瘤内,凝血诱导蛋白组织因子(TF)是血液凝固的主要引发剂,由于其对肿瘤的高凝性和介导的相互作用的介导作用,已被证明在肿瘤的血源性转移中起着至关重要的作用。血小板和肿瘤细胞。因此,靶向肿瘤相关的TF对于癌症患者的抗转移治疗和预防血栓形成并发症具有巨大的治疗潜力。在本文中,我们报道了一种新型的基于肽的纳米颗粒,其靶向小干扰RNA(siRNA)的递送和释放进入肿瘤部位,以沉默肿瘤相关TF的表达。我们表明抑制肿瘤细胞中的TF表达可在体外阻断肿瘤细胞周围的血小板粘附。静脉内施用的肿瘤细胞(即模拟循环肿瘤细胞[CTC])中TF表达的下调阻止了CTC周围的血小板粘附并降低了肺中CTC的存活率。在乳腺癌小鼠模型中,含siRNA的纳米颗粒可有效减弱肿瘤微环境中的TF表达,并显着减少实验性肺转移模型和荷瘤小鼠的肺转移量。更重要的是,该策略通过减少凝血酶-抗凝血酶复合物(TAT)和活化血小板的生成来逆转荷瘤小鼠的高凝状态,两者都是TF的下游产物。我们的研究描述了一种有前途的方法来对抗转移和预防癌症相关的血栓形成,
更新日期:2019-06-07
中文翻译:

组织因子的肿瘤特异性沉默抑制转移并预防癌症相关的高凝性
在肿瘤内,凝血诱导蛋白组织因子(TF)是血液凝固的主要引发剂,由于其对肿瘤的高凝性和介导的相互作用的介导作用,已被证明在肿瘤的血源性转移中起着至关重要的作用。血小板和肿瘤细胞。因此,靶向肿瘤相关的TF对于癌症患者的抗转移治疗和预防血栓形成并发症具有巨大的治疗潜力。在本文中,我们报道了一种新型的基于肽的纳米颗粒,其靶向小干扰RNA(siRNA)的递送和释放进入肿瘤部位,以沉默肿瘤相关TF的表达。我们表明抑制肿瘤细胞中的TF表达可在体外阻断肿瘤细胞周围的血小板粘附。静脉内施用的肿瘤细胞(即模拟循环肿瘤细胞[CTC])中TF表达的下调阻止了CTC周围的血小板粘附并降低了肺中CTC的存活率。在乳腺癌小鼠模型中,含siRNA的纳米颗粒可有效减弱肿瘤微环境中的TF表达,并显着减少实验性肺转移模型和荷瘤小鼠的肺转移量。更重要的是,该策略通过减少凝血酶-抗凝血酶复合物(TAT)和活化血小板的生成来逆转荷瘤小鼠的高凝状态,两者都是TF的下游产物。我们的研究描述了一种有前途的方法来对抗转移和预防癌症相关的血栓形成,































 京公网安备 11010802027423号
京公网安备 11010802027423号