当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decahydroquinoline Ring 13C NMR Spectroscopic Patterns for the Stereochemical Elucidation of Phlegmarine-Type Lycopodium Alkaloids: Synthesis of (-)-Serralongamine A and Structural Reassignment and Synthesis of (-)-Huperzine K and (-)-Huperzine M (Lycoposerramine Y).
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-06-05 , DOI: 10.1021/acs.jnatprod.9b00071 Caroline Bosch 1 , Ben Bradshaw 1 , Josep Bonjoch 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-06-05 , DOI: 10.1021/acs.jnatprod.9b00071 Caroline Bosch 1 , Ben Bradshaw 1 , Josep Bonjoch 1
Affiliation
|
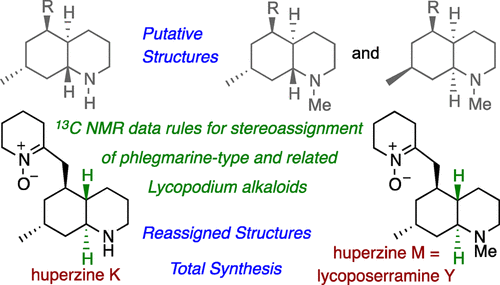
Analysis of 13C NMR spectroscopic data of the phlegmarine subset of Lycopodium alkaloids revealed spectral patterns that allowed the stereochemical arrangement of the four stereogenic carbons in the decahydroquinoline core to be established. A relatively simple predictive set of chemical shift combinations is reported, providing a tool for the challenging stereochemical assignment of phlegmarine-type alkaloids. Based on the chemical shifts in their NMR spectroscopic profiles, the alkaloids huperzine K and huperzine M, formally reported as cis derivatives, were structurally reassigned as trans-decahydroquinolines. The NMR spectroscopic data for huperzine M were identical to those reported for lycoposerramine Y and, hence, also implied the configurational reassignment of the latter. The revised structures of the above alkaloids were confirmed by enantioselective total synthesis. Additionally, the synthesis of (-)-serralongamine A via a common intermediate precursor is reported.
中文翻译:

十氢喹啉环13C NMR光谱模式用于立体鉴定Phmarmarine型Lypopodium生物碱:(-)-Serralongamine A的合成以及(-)-Huperzine K和(-)-Huperzine M(Lycoposerramine Y)的结构再分配和合成。
对石蒜碱生物碱的化石棉亚群的13 C NMR光谱数据进行分析后发现,光谱图谱可确定十氢喹啉核心中四个立体异构碳的立体化学排列。据报道,相对简单的化学位移组合预测集,为富勒麻碱型生物碱的立体化学分配提供了挑战。根据其NMR光谱图的化学变化,正式报告为顺式衍生物的生物碱石杉碱K和石杉碱M在结构上被重新指定为反式十氢喹啉。石杉碱M的NMR光谱数据与lyposeposerramine Y报道的数据相同,因此也暗示了后者的构型重新分配。通过对映选择性的全合成证实了上述生物碱的修饰结构。另外,报告了通过共同的中间体前体合成(-)-serralongamineA。
更新日期:2019-06-05
中文翻译:

十氢喹啉环13C NMR光谱模式用于立体鉴定Phmarmarine型Lypopodium生物碱:(-)-Serralongamine A的合成以及(-)-Huperzine K和(-)-Huperzine M(Lycoposerramine Y)的结构再分配和合成。
对石蒜碱生物碱的化石棉亚群的13 C NMR光谱数据进行分析后发现,光谱图谱可确定十氢喹啉核心中四个立体异构碳的立体化学排列。据报道,相对简单的化学位移组合预测集,为富勒麻碱型生物碱的立体化学分配提供了挑战。根据其NMR光谱图的化学变化,正式报告为顺式衍生物的生物碱石杉碱K和石杉碱M在结构上被重新指定为反式十氢喹啉。石杉碱M的NMR光谱数据与lyposeposerramine Y报道的数据相同,因此也暗示了后者的构型重新分配。通过对映选择性的全合成证实了上述生物碱的修饰结构。另外,报告了通过共同的中间体前体合成(-)-serralongamineA。































 京公网安备 11010802027423号
京公网安备 11010802027423号