当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of the Reaction Mechanism of Alkylaromatics Transalkylation by Means of Molecular Confinement Effects Associated to Zeolite Channel Architecture
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-06-05 00:00:00 , DOI: 10.1021/acscatal.9b00763
Vicente J. Margarit 1 , Mogahid Osman 2 , Sulaiman Al-Khattaf 2 , Cristina Martínez 1 , Mercedes Boronat 1 , Avelino Corma 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-06-05 00:00:00 , DOI: 10.1021/acscatal.9b00763
Vicente J. Margarit 1 , Mogahid Osman 2 , Sulaiman Al-Khattaf 2 , Cristina Martínez 1 , Mercedes Boronat 1 , Avelino Corma 1
Affiliation
|
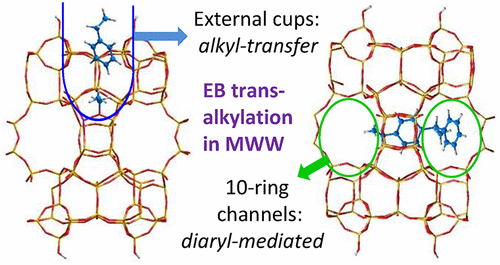
Transalkylation of alkylaromatics catalyzed by acid zeolites is a process widely employed in the petrochemical industry for upgrading aromatic fractions. The reaction mechanism is complex as it can proceed either by intermolecular alkyl-transfer involving dealkylation-alkylation steps with surface alkoxy species as reaction intermediates or through the formation of bulkier diaryl intermediates. We have investigated how the possible formation of such bulky intermediates in the microporous channel system of different zeolite structures, together with their stabilization by confinement effects, can determine the preferential mechanism and, therefore, the selectivity of ethylbenzene disproportionation into benzene and diethylbenzene. For testing the concept, four zeolites, MCM-22 (3D MWW) with 10R pores, 12R cavities and external 12R hemicavities or “cups”, DS-ITQ-2, (2D MWW) with the same 10R channels as MCM-22, no 12R cavities and much larger proportion of external “cups”, a 10R ZSM-5 (MFI) and a 12R mordenite (MOR) have been used. The higher activity of DS-ITQ-2 and MCM-22 as compared to ZSM-5 at low temperature (573 K) and the high selectivity to diethylbenzene of the bidimensional material under all reaction conditions considered have been explained by means of DFT calculations. Contrary to what could be expected according to the available space at the external “cups” and at the 10R channels of the MWW structure, the bulkier diaryl intermediates are better stabilized within the 10R channel system than at the “cups” open at the external surface of the MWW materials. We show from this perspective how the channel structure and molecular confinement stabilization also explain the operating reaction mechanism in ZSM-5 and mordenite.
中文翻译:

通过与分子筛通道结构相关的分子限制效应来控制烷基链芳烃烷基转移的反应机理
酸性沸石催化的烷基芳烃的烷基转移是石油化学工业中广泛用于提高芳烃馏分含量的方法。该反应机理是复杂的,因为它可以通过分子间的烷基转移来进行,该分子间的烷基转移包括以表面烷氧基为反应中间体的脱烷基化-烷基化步骤,或者通过形成较大的二芳基中间体来进行。我们已经研究了在不同沸石结构的微孔通道系统中可能形成的这种大体积中间体,以及它们在封闭作用下的稳定作用,如何确定优先机理,从而确定了乙苯歧化成苯和二乙苯的选择性。为了测试该概念,使用了四种具有10R孔的沸石MCM-22(3D MWW),12R腔和外部12R半腔或“杯”,DS-ITQ-2(2D MWW),具有与MCM-22相同的10R通道,没有12R腔,且外部“杯”的比例更大,一个10R ZSM-5( MFI)和12R丝光沸石(MOR)已被使用。DS-ITQ-2和MCM-22在低温(573 K)下与ZSM-5相比具有更高的活性,并且在所考虑的所有反应条件下该二维材料对二乙苯的选择性都已通过DFT计算得到了解释。与根据外部“杯”和MWW结构的10R通道上的可用空间所预期的相反,在10R通道系统内,比在外表面打开的“杯”上,较大的二芳基中间体更好地稳定。 MWW材料。
更新日期:2019-06-05
中文翻译:

通过与分子筛通道结构相关的分子限制效应来控制烷基链芳烃烷基转移的反应机理
酸性沸石催化的烷基芳烃的烷基转移是石油化学工业中广泛用于提高芳烃馏分含量的方法。该反应机理是复杂的,因为它可以通过分子间的烷基转移来进行,该分子间的烷基转移包括以表面烷氧基为反应中间体的脱烷基化-烷基化步骤,或者通过形成较大的二芳基中间体来进行。我们已经研究了在不同沸石结构的微孔通道系统中可能形成的这种大体积中间体,以及它们在封闭作用下的稳定作用,如何确定优先机理,从而确定了乙苯歧化成苯和二乙苯的选择性。为了测试该概念,使用了四种具有10R孔的沸石MCM-22(3D MWW),12R腔和外部12R半腔或“杯”,DS-ITQ-2(2D MWW),具有与MCM-22相同的10R通道,没有12R腔,且外部“杯”的比例更大,一个10R ZSM-5( MFI)和12R丝光沸石(MOR)已被使用。DS-ITQ-2和MCM-22在低温(573 K)下与ZSM-5相比具有更高的活性,并且在所考虑的所有反应条件下该二维材料对二乙苯的选择性都已通过DFT计算得到了解释。与根据外部“杯”和MWW结构的10R通道上的可用空间所预期的相反,在10R通道系统内,比在外表面打开的“杯”上,较大的二芳基中间体更好地稳定。 MWW材料。

































 京公网安备 11010802027423号
京公网安备 11010802027423号