当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogen Sublimation as a Driver of Chemistry in Pluto-Analog Laboratory Ices: Formation of Carbon Suboxide (C3O2) and Various Salts
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-06-06 00:00:00 , DOI: 10.1021/acsearthspacechem.9b00005 Kamil B. Stelmach 1, 2 , Yukiko Y. Yarnall 1 , Paul D. Cooper 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-06-06 00:00:00 , DOI: 10.1021/acsearthspacechem.9b00005 Kamil B. Stelmach 1, 2 , Yukiko Y. Yarnall 1 , Paul D. Cooper 1
Affiliation
|
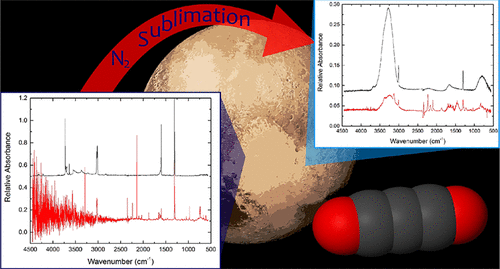
A number of planetary bodies, including Triton and Pluto, and a number of Kuiper Belt objects contain nitrogen ices on their surfaces. Nitrogen ices were also used in laboratory experiments as a matrix isolation material before noble gases could be condensed. Planetary bodies with nitrogen ices then may act as giant matrix isolation experiments, trapping reactive species onto the surface and concentrating them. Upon sublimation, these reactive species are much more likely to encounter each other or another molecule to react with. A pilot study was conducted to test the feasibility of testing the chemistry occurring during the sublimation of nitrogen ices. A high vacuum laboratory setup was used to create ices at ∼6 K (±0.5 K). Ices were deposited under microwave radiation to create radicals to simulate what might be present in the tenuous atmospheres of these planetary bodies. The ices consisted of a mixture of 1:1:100 carbon source + H2O + N2, where the carbon source was either CO or CH4. Reagents and products were primarily identified using FTIR and UV–vis transmission spectroscopy. Once the predominantly N2 ice was characterized with the spectroscopic techniques, the N2 was sublimated to create a H2O ice, and then this ice was characterized using the aforementioned techniques. One completely new product was observed, namely, carbon suboxide (C3O2), and a couple products identified in the nitrogen ice formed various salts. Future work could make use of multiple sublimation steps and other astrochemically relevant matrices and introduce more astrophysically relevant sources of radiation like electron beams or UV irradiation.
中文翻译:

氮升华是冥想模拟实验室冰中化学的驱动因素:二氧化碳(C 3 O 2)和各种盐的形成
许多行星体,包括海卫一和冥王星,以及许多柯伊伯带天体,其表面都含有氮冰。在稀有气体可以冷凝之前,氮气冰还用于实验室实验,作为基质隔离材料。然后,带有氮冰的行星体可以充当巨大的基质隔离实验,将反应性物种捕获到表面上并进行浓缩。在升华时,这些反应性物种更可能彼此相遇或与另一分子发生反应。进行了一项中试研究,以测试测试在氮气冰升华过程中发生的化学反应的可行性。使用高真空实验室设置以约6 K(±0.5 K)的高度结冰。冰在微波辐射下沉积,产生自由基,以模拟这些行星体的脆弱大气中可能存在的物质。冰由1:1:100碳源+ H的混合物组成2 O + N 2,其中碳源为CO或CH 4。试剂和产品主要使用FTIR和UV-vis透射光谱法进行鉴定。一旦主要的N 2冰用光谱技术表征,就将N 2升华以产生H 2 O冰,然后使用前述技术表征该冰。观察到一种全新的产物,即低碳(C 3 O 2),在氮气冰中鉴定出的几种产物形成了各种盐。未来的工作可能会利用多个升华步骤和其他与天体化学有关的基质,并引入更多与天文学有关的辐射源,例如电子束或紫外线辐射。
更新日期:2019-06-06
中文翻译:

氮升华是冥想模拟实验室冰中化学的驱动因素:二氧化碳(C 3 O 2)和各种盐的形成
许多行星体,包括海卫一和冥王星,以及许多柯伊伯带天体,其表面都含有氮冰。在稀有气体可以冷凝之前,氮气冰还用于实验室实验,作为基质隔离材料。然后,带有氮冰的行星体可以充当巨大的基质隔离实验,将反应性物种捕获到表面上并进行浓缩。在升华时,这些反应性物种更可能彼此相遇或与另一分子发生反应。进行了一项中试研究,以测试测试在氮气冰升华过程中发生的化学反应的可行性。使用高真空实验室设置以约6 K(±0.5 K)的高度结冰。冰在微波辐射下沉积,产生自由基,以模拟这些行星体的脆弱大气中可能存在的物质。冰由1:1:100碳源+ H的混合物组成2 O + N 2,其中碳源为CO或CH 4。试剂和产品主要使用FTIR和UV-vis透射光谱法进行鉴定。一旦主要的N 2冰用光谱技术表征,就将N 2升华以产生H 2 O冰,然后使用前述技术表征该冰。观察到一种全新的产物,即低碳(C 3 O 2),在氮气冰中鉴定出的几种产物形成了各种盐。未来的工作可能会利用多个升华步骤和其他与天体化学有关的基质,并引入更多与天文学有关的辐射源,例如电子束或紫外线辐射。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号