当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genetically Encoding Photocaged Quinone Methide to Multitarget Protein Residues Covalently in Vivo
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-06-04 , DOI: 10.1021/jacs.9b01738 Jun Liu 1 , Shanshan Li 1, 2 , Nayyar A Aslam 3 , Feng Zheng 3 , Bing Yang 1 , Rujin Cheng 4 , Nanxi Wang 1 , Sharon Rozovsky 4 , Peng G Wang 2 , Qian Wang 3 , Lei Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-06-04 , DOI: 10.1021/jacs.9b01738 Jun Liu 1 , Shanshan Li 1, 2 , Nayyar A Aslam 3 , Feng Zheng 3 , Bing Yang 1 , Rujin Cheng 4 , Nanxi Wang 1 , Sharon Rozovsky 4 , Peng G Wang 2 , Qian Wang 3 , Lei Wang 1
Affiliation
|
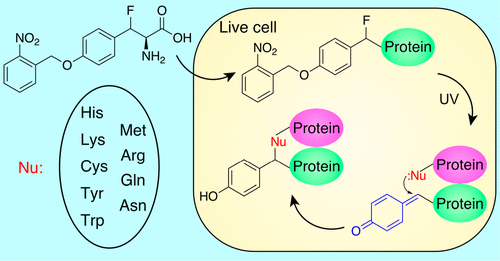
Genetically introducing covalent bonds into proteins in vivo with residue specificity is affording innovative ways for protein research and engineering, yet latent bioreactive unnatural amino acids (Uaas) genetically encoded to date react with one to few natural residues only, limiting the variety of proteins and the scope of applications amenable to this technology. Here we report the genetic encoding of (2 R)-2-amino-3-fluoro-3-(4-((2-nitrobenzyl)oxy) phenyl) propanoic acid (FnbY) in Escherichia coli and mammalian cells. Upon photoactivation, FnbY generated a reactive quinone methide (QM), which selectively reacted with nine natural amino acid residues placed in proximity in proteins directly in live cells. In addition to Cys, Lys, His, and Tyr, photoactivated FnbY also reacted with Trp, Met, Arg, Asn, and Gln, which are inaccessible with existing latent bioreactive Uaas. FnbY thus dramatically expanded the number of residues for covalent targeting in vivo. QM has longer half-life than the intermediates of conventional photo-cross-linking Uaas, and FnbY exhibited cross-linking efficiency higher than p-azido-phenylalanine. The photoactivatable and multitargeting reactivity of FnbY with selectivity toward nucleophilic residues will be valuable for addressing diverse proteins and broadening the scope of applications through exploiting covalent bonding in vivo for chemical biology, biotherapeutics, and protein engineering.
中文翻译:

在体内将光笼化醌甲基化物基因编码为多靶点蛋白质残基
在体内将具有残基特异性的共价键遗传引入蛋白质为蛋白质研究和工程提供了创新方法,但迄今为止遗传编码的潜在生物反应性非天然氨基酸 (Uaas) 仅与一到几个天然残基发生反应,限制了蛋白质的种类和适用于该技术的应用范围。在这里我们报告了 (2 R)-2-amino-3-fluoro-3-(4-((2-nitrobenzyl)oxy) phenyl) 丙酸 (FnbY) 在大肠杆菌和哺乳动物细胞中的遗传编码。光活化后,FnbY 产生反应性醌甲基化物 (QM),它选择性地与直接在活细胞中的蛋白质中放置的九个天然氨基酸残基反应。除了 Cys、Lys、His 和 Tyr,光活化的 FnbY 还与 Trp、Met、Arg、Asn 和 Gln 反应,这是现有潜在生物反应性 Uaas 无法访问的。因此,FnbY 显着增加了体内共价靶向的残基数量。QM 比传统光交联 Uaas 的中间体具有更长的半衰期,FnbY 的交联效率高于对叠氮基苯丙氨酸。FnbY 的可光活化和多靶点反应性对亲核残基具有选择性,这对于解决多种蛋白质和通过在体内开发共价键用于化学生物学、生物治疗学和蛋白质工程来拓宽应用范围是有价值的。和 FnbY 表现出高于对叠氮基苯丙氨酸的交联效率。FnbY 的可光活化和多靶点反应性对亲核残基具有选择性,这对于解决多种蛋白质和通过在体内开发共价键用于化学生物学、生物治疗学和蛋白质工程来拓宽应用范围是有价值的。和 FnbY 表现出高于对叠氮基苯丙氨酸的交联效率。FnbY 的可光活化和多靶点反应性对亲核残基具有选择性,这对于解决多种蛋白质和通过在体内开发共价键用于化学生物学、生物治疗学和蛋白质工程来拓宽应用范围是有价值的。
更新日期:2019-06-04
中文翻译:

在体内将光笼化醌甲基化物基因编码为多靶点蛋白质残基
在体内将具有残基特异性的共价键遗传引入蛋白质为蛋白质研究和工程提供了创新方法,但迄今为止遗传编码的潜在生物反应性非天然氨基酸 (Uaas) 仅与一到几个天然残基发生反应,限制了蛋白质的种类和适用于该技术的应用范围。在这里我们报告了 (2 R)-2-amino-3-fluoro-3-(4-((2-nitrobenzyl)oxy) phenyl) 丙酸 (FnbY) 在大肠杆菌和哺乳动物细胞中的遗传编码。光活化后,FnbY 产生反应性醌甲基化物 (QM),它选择性地与直接在活细胞中的蛋白质中放置的九个天然氨基酸残基反应。除了 Cys、Lys、His 和 Tyr,光活化的 FnbY 还与 Trp、Met、Arg、Asn 和 Gln 反应,这是现有潜在生物反应性 Uaas 无法访问的。因此,FnbY 显着增加了体内共价靶向的残基数量。QM 比传统光交联 Uaas 的中间体具有更长的半衰期,FnbY 的交联效率高于对叠氮基苯丙氨酸。FnbY 的可光活化和多靶点反应性对亲核残基具有选择性,这对于解决多种蛋白质和通过在体内开发共价键用于化学生物学、生物治疗学和蛋白质工程来拓宽应用范围是有价值的。和 FnbY 表现出高于对叠氮基苯丙氨酸的交联效率。FnbY 的可光活化和多靶点反应性对亲核残基具有选择性,这对于解决多种蛋白质和通过在体内开发共价键用于化学生物学、生物治疗学和蛋白质工程来拓宽应用范围是有价值的。和 FnbY 表现出高于对叠氮基苯丙氨酸的交联效率。FnbY 的可光活化和多靶点反应性对亲核残基具有选择性,这对于解决多种蛋白质和通过在体内开发共价键用于化学生物学、生物治疗学和蛋白质工程来拓宽应用范围是有价值的。































 京公网安备 11010802027423号
京公网安备 11010802027423号