当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formulation and preclinical evaluation of a toll-like receptor 7/8 agonist as an anti-tumoral immunomodulator.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-06-04 , DOI: 10.1016/j.jconrel.2019.06.003 Ruolin Lu 1 , Chad Groer 2 , Peter A Kleindl 1 , K Ryan Moulder 1 , Aric Huang 1 , Jordan R Hunt 1 , Shuang Cai 3 , Daniel J Aires 4 , Cory Berkland 5 , M Laird Forrest 3
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-06-04 , DOI: 10.1016/j.jconrel.2019.06.003 Ruolin Lu 1 , Chad Groer 2 , Peter A Kleindl 1 , K Ryan Moulder 1 , Aric Huang 1 , Jordan R Hunt 1 , Shuang Cai 3 , Daniel J Aires 4 , Cory Berkland 5 , M Laird Forrest 3
Affiliation
|
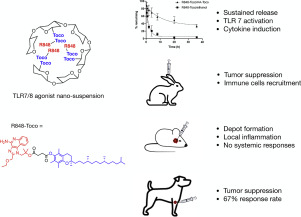
The toll-like receptor 7 and 8 (TLR7/8) agonist Resiquimod (R848) has been recognized as a promising immunostimulator for the treatment of cutaneous cancers in multiple clinical trials. However, systemic administration of R848 often results in strong immune-related toxicities while having limited therapeutic effects to the tumor. In the present study, a prodrug-based nanocarrier delivery system was developed that exhibited high therapeutic efficiency. R848 was conjugated to α-tocopherol to constitute an R848-Toco prodrug, followed by formulating with a tocopherol-modified hyaluronic acid (HA-Toco) as a polymeric nano-suspension. In vitro evaluation showed that the delivery system prolonged the release kinetics while maintaining TLR agonist activities. When administered subcutaneously, the nano-suspension formed a depot at the injection site, inducing localized immune responses without systemic expansion. This formulation also suppressed tumor growth and recruited immune cells to the tumor in a murine model of head and neck cancer. In a preclinical canine study of spontaneous mast cell tumors, the treatment led to a 67% response rate (three partial remissions and one complete remission).
中文翻译:

Toll样受体7/8激动剂作为抗肿瘤免疫调节剂的配制和临床前评价。
Toll样受体7和8(TLR7 / 8)激动剂Resiquimod(R848)在多种临床试验中被公认为治疗皮肤癌的有希望的免疫刺激剂。但是,R848的全身给药通常会导致强烈的免疫相关毒性,同时对肿瘤的治疗作用有限。在本研究中,开发了一种基于前药的纳米载体递送系统,该系统表现出很高的治疗效率。将R848与α-生育酚缀合以构成R848-Toco前药,然后与生育酚改性的透明质酸(HA-Toco)一起配制为聚合物纳米悬浮液。体外评估表明,该递送系统在维持TLR激动剂活性的同时延长了释放动力学。皮下给药时,纳米混悬剂在注射部位形成了长效制剂,诱导局部免疫反应而不全身性扩张。在头颈癌的鼠模型中,该制剂还抑制了肿瘤的生长并募集了免疫细胞到肿瘤。在临床上对自发性肥大细胞肿瘤的犬类研究中,该治疗导致67%的缓解率(三部分缓解和一完全缓解)。
更新日期:2019-06-04
中文翻译:

Toll样受体7/8激动剂作为抗肿瘤免疫调节剂的配制和临床前评价。
Toll样受体7和8(TLR7 / 8)激动剂Resiquimod(R848)在多种临床试验中被公认为治疗皮肤癌的有希望的免疫刺激剂。但是,R848的全身给药通常会导致强烈的免疫相关毒性,同时对肿瘤的治疗作用有限。在本研究中,开发了一种基于前药的纳米载体递送系统,该系统表现出很高的治疗效率。将R848与α-生育酚缀合以构成R848-Toco前药,然后与生育酚改性的透明质酸(HA-Toco)一起配制为聚合物纳米悬浮液。体外评估表明,该递送系统在维持TLR激动剂活性的同时延长了释放动力学。皮下给药时,纳米混悬剂在注射部位形成了长效制剂,诱导局部免疫反应而不全身性扩张。在头颈癌的鼠模型中,该制剂还抑制了肿瘤的生长并募集了免疫细胞到肿瘤。在临床上对自发性肥大细胞肿瘤的犬类研究中,该治疗导致67%的缓解率(三部分缓解和一完全缓解)。















































 京公网安备 11010802027423号
京公网安备 11010802027423号