当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting Carvone To Demonstrate Both Stereocontrol and Regiocontrol: 1,2- vs 1,4-Addition of Grignard Reagents and Organocuprates
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.jchemed.7b00892 Thai Phat Truong 1 , Sophia J. Bailey 1 , Alexandra E. Golliher 1 , Erika Y. Monroy 1 , Uttar K. Shrestha 1 , William A. Maio 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.jchemed.7b00892 Thai Phat Truong 1 , Sophia J. Bailey 1 , Alexandra E. Golliher 1 , Erika Y. Monroy 1 , Uttar K. Shrestha 1 , William A. Maio 1
Affiliation
|
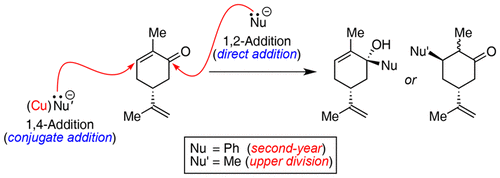
The ability of certain organometallic reagents to react via 1,2- or 1,4-addition to an α,β-unsaturated ketone is a fundamental example of regioselectivity at the second-year undergraduate organic level. The following two experiments were designed to demonstrate this preference by exploiting carvone as an inexpensive chiral, nonracemic substrate. The first, intended for a typical undergraduate audience, makes use of phenylmagnesium bromide; the second calls for the manufacture of lithium dimethylcuprate from a stock solution of methyl lithium and copper(I) iodide and is envisioned to be carried out by upper-division students. Importantly, due to the chiral nature of carvone, these addition reactions are highly stereoselective and will provide an opportunity for students to revisit stereochemistry. Also discussed are several thoughts on assessment of student learning as well as an easy to adopt protocol that details reaction setup, aqueous workup, purification, waste management, and the analysis of products using 1H NMR spectroscopy.
中文翻译:

利用香芹酮来显示立体控制和区域控制:格氏试剂和有机化合物的1,2-vs,1,4-vs
某些有机金属试剂通过1,2-或1,4-加成至α,β-不饱和酮反应的能力是在大学本科二年级时区域选择性的一个基本例子。设计以下两个实验以通过利用香芹酮作为廉价的手性,非外消旋底物来证明这种偏好。第一种针对的是典型的本科生,使用了苯基溴化镁。第二个要求由甲基锂和碘化铜(I)的储备溶液生产二甲基铜锂,预计由高年级学生进行。重要的是,由于香芹酮的手性,这些加成反应具有很高的立体选择性,并为学生提供了重温立体化学的机会。1 H NMR光谱。
更新日期:2018-02-19
中文翻译:

利用香芹酮来显示立体控制和区域控制:格氏试剂和有机化合物的1,2-vs,1,4-vs
某些有机金属试剂通过1,2-或1,4-加成至α,β-不饱和酮反应的能力是在大学本科二年级时区域选择性的一个基本例子。设计以下两个实验以通过利用香芹酮作为廉价的手性,非外消旋底物来证明这种偏好。第一种针对的是典型的本科生,使用了苯基溴化镁。第二个要求由甲基锂和碘化铜(I)的储备溶液生产二甲基铜锂,预计由高年级学生进行。重要的是,由于香芹酮的手性,这些加成反应具有很高的立体选择性,并为学生提供了重温立体化学的机会。1 H NMR光谱。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号