当前位置:
X-MOL 学术
›
Nat. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression.
Nature Immunology ( IF 27.7 ) Pub Date : 2019-06-03 , DOI: 10.1038/s41590-019-0400-7 Qingsong Hu , Youqiong Ye , Li-Chuan Chan , Yajuan Li , Ke Liang , Aifu Lin , Sergey D. Egranov , Yaohua Zhang , Weiya Xia , Jing Gong , Yinghong Pan , Sujash S. Chatterjee , Jun Yao , Kurt W. Evans , Tina K. Nguyen , Peter K. Park , Jiewei Liu , Cristian Coarfa , Sri Ramya Donepudi , Vasanta Putluri , Nagireddy Putluri , Arun Sreekumar , Chandrashekar R. Ambati , David H. Hawke , Jeffrey R. Marks , Preethi H. Gunaratne , Abigail S. Caudle , Aysegul A. Sahin , Gabriel N. Hortobagyi , Funda Meric-Bernstam , Lieping Chen , Dihua Yu , Mien-Chie Hung , Michael A. Curran , Leng Han , Chunru Lin , Liuqing Yang
Nature Immunology ( IF 27.7 ) Pub Date : 2019-06-03 , DOI: 10.1038/s41590-019-0400-7 Qingsong Hu , Youqiong Ye , Li-Chuan Chan , Yajuan Li , Ke Liang , Aifu Lin , Sergey D. Egranov , Yaohua Zhang , Weiya Xia , Jing Gong , Yinghong Pan , Sujash S. Chatterjee , Jun Yao , Kurt W. Evans , Tina K. Nguyen , Peter K. Park , Jiewei Liu , Cristian Coarfa , Sri Ramya Donepudi , Vasanta Putluri , Nagireddy Putluri , Arun Sreekumar , Chandrashekar R. Ambati , David H. Hawke , Jeffrey R. Marks , Preethi H. Gunaratne , Abigail S. Caudle , Aysegul A. Sahin , Gabriel N. Hortobagyi , Funda Meric-Bernstam , Lieping Chen , Dihua Yu , Mien-Chie Hung , Michael A. Curran , Leng Han , Chunru Lin , Liuqing Yang
|
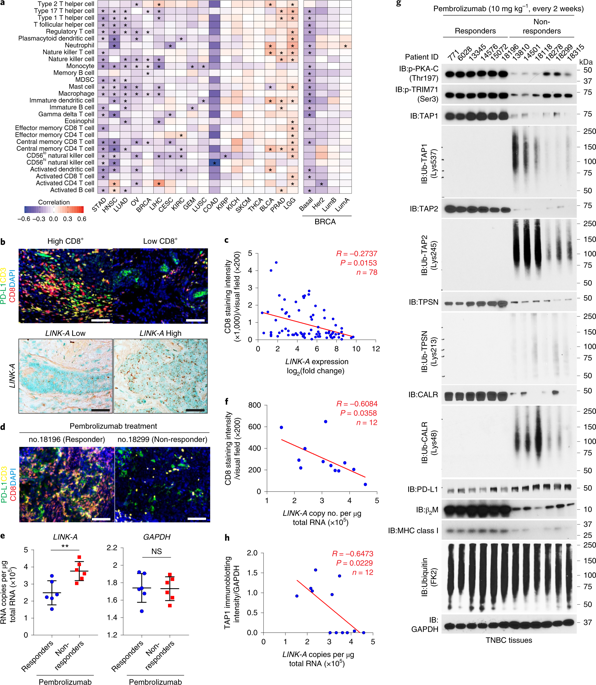
How tumor cells genetically lose antigenicity and evade immune checkpoints remains largely elusive. We report that tissue-specific expression of the human long noncoding RNA LINK-A in mouse mammary glands initiates metastatic mammary gland tumors, which phenotypically resemble human triple-negative breast cancer (TNBC). LINK-A expression facilitated crosstalk between phosphatidylinositol-(3,4,5)-trisphosphate and inhibitory G-protein-coupled receptor (GPCR) pathways, attenuating protein kinase A-mediated phosphorylation of the E3 ubiquitin ligase TRIM71. Consequently, LINK-A expression enhanced K48-polyubiquitination-mediated degradation of the antigen peptide-loading complex (PLC) and intrinsic tumor suppressors Rb and p53. Treatment with LINK-A locked nucleic acids or GPCR antagonists stabilized the PLC components, Rb and p53, and sensitized mammary gland tumors to immune checkpoint blockers. Patients with programmed ccll death protein-1(PD-1) blockade-resistant TNBC exhibited elevated LINK-A levels and downregulated PLC components. Hence we demonstrate lncRNA-dependent downregulation of antigenicity and intrinsic tumor suppression, which provides the basis for developing combinational immunotherapy treatment regimens and early TNBC prevention.
中文翻译:

致癌 lncRNA 下调癌细胞抗原呈递和内在肿瘤抑制。
肿瘤细胞如何在遗传上失去抗原性并逃避免疫检查点仍然在很大程度上难以捉摸。我们报道了人类长链非编码 RNA LINK-A 在小鼠乳腺中的组织特异性表达引发了转移性乳腺肿瘤,其表型类似于人类三阴性乳腺癌 (TNBC)。LINK-A 表达促进了磷脂酰肌醇-(3,4,5)-三磷酸和抑制性 G 蛋白偶联受体 (GPCR) 通路之间的串扰,减弱了蛋白激酶 A 介导的 E3 泛素连接酶 TRIM71 的磷酸化。因此,LINK-A 表达增强了 K48 多聚泛素化介导的抗原肽负载复合物 (PLC) 和内在肿瘤抑制因子 Rb 和 p53 的降解。用 LINK-A 锁核酸或 GPCR 拮抗剂处理可稳定 PLC 成分、Rb 和 p53,并使乳腺肿瘤对免疫检查点阻滞剂敏感。具有程序性 ccll 死亡蛋白-1 (PD-1) 阻断抗性的 TNBC 患者表现出升高的 LINK-A 水平和下调的 PLC 组件。因此,我们证明了 lncRNA 依赖性下调抗原性和内在肿瘤抑制,这为开发联合免疫治疗方案和早期 TNBC 预防提供了基础。
更新日期:2019-06-04
中文翻译:

致癌 lncRNA 下调癌细胞抗原呈递和内在肿瘤抑制。
肿瘤细胞如何在遗传上失去抗原性并逃避免疫检查点仍然在很大程度上难以捉摸。我们报道了人类长链非编码 RNA LINK-A 在小鼠乳腺中的组织特异性表达引发了转移性乳腺肿瘤,其表型类似于人类三阴性乳腺癌 (TNBC)。LINK-A 表达促进了磷脂酰肌醇-(3,4,5)-三磷酸和抑制性 G 蛋白偶联受体 (GPCR) 通路之间的串扰,减弱了蛋白激酶 A 介导的 E3 泛素连接酶 TRIM71 的磷酸化。因此,LINK-A 表达增强了 K48 多聚泛素化介导的抗原肽负载复合物 (PLC) 和内在肿瘤抑制因子 Rb 和 p53 的降解。用 LINK-A 锁核酸或 GPCR 拮抗剂处理可稳定 PLC 成分、Rb 和 p53,并使乳腺肿瘤对免疫检查点阻滞剂敏感。具有程序性 ccll 死亡蛋白-1 (PD-1) 阻断抗性的 TNBC 患者表现出升高的 LINK-A 水平和下调的 PLC 组件。因此,我们证明了 lncRNA 依赖性下调抗原性和内在肿瘤抑制,这为开发联合免疫治疗方案和早期 TNBC 预防提供了基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号