当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Screening of the Structure of Americium Extractants Based on a 2,2’‐Bipyridyl Scaffold: a Simple Way to a N2,O2‐Tetradentate Ligands Library for Rational Design of An/Ln Extractants
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-02-20 , DOI: 10.1002/slct.201702741 Nataliya E. Borisova 1, 2 , Alexey V. Ivanov 1 , Petr I. Matveev 1 , Anastasiya A. Smirnova 1 , Elena V. Belova 3 , Stepan N. Kalmykov 1, 3 , Boris F. Myasoedov 3
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-02-20 , DOI: 10.1002/slct.201702741 Nataliya E. Borisova 1, 2 , Alexey V. Ivanov 1 , Petr I. Matveev 1 , Anastasiya A. Smirnova 1 , Elena V. Belova 3 , Stepan N. Kalmykov 1, 3 , Boris F. Myasoedov 3
Affiliation
|
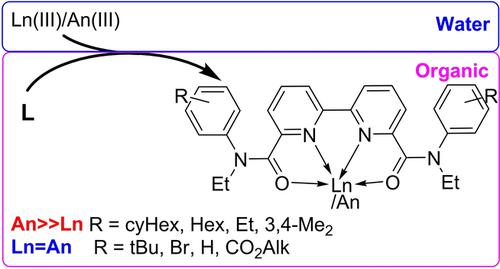
A library of N‐ethylanilides of 2,2′‐bipyridine‐6,6′‐dicarboxylic acids were synthesized to evaluate the influence of the side chains on the extraction properties. The structure of the various diamides is studied in solid state by X‐ray and in solution by 2D NMR. In the solid‐state, the diamides featured flat Cs geometry irrespective of the nature and position of the substituents. Carboxylic groups in the crystals shifted from the residing planes of both pyridine and aromatic anilide rings indicating disruption of the conjugated system. The solution behaviour is substituent dependent. Only three types of relative orientations of amidic fragments and pyridine rings were found in solutions of diamides depending on the nature of the amidic side chain substituents. The conformations of the diamides mostly depended on the size of the substituent and less on their electronic properties. Solubility of the diamides and their metal extraction properties in relation to americium and europium ions were studied the diamides, bearing substituents in pyridine rings and in phenyl ring of diamide moiety (one or two alkyl‐, alkoxy‐, fluorine‐, carboxylic‐groups). The symmetrical substitution in the pyridine moiety critically diminished both solubility and metal extraction. Metal ions recovery strongly depended on the substitution in the anilidic moiety; distribution coefficients depended more on the spatial arrangement of the substituents and to a lesser extent on their electronic properties.
中文翻译:

基于2,2'-联吡啶骨架的A提取剂结构的筛选:一种用于N / O2-四齿配体库的简单方法,用于合理设计An / Ln提取剂
合成了一个2,2'-联吡啶-6,6'-二羧酸N-乙基苯胺的文库,以评估侧链对提取性能的影响。各种二酰胺的结构在X射线下以固态研究,在溶液中通过2D NMR研究。在固态状态下,二酰胺具有平坦的Cs几何形状,而与取代基的性质和位置无关。晶体中的羧基从吡啶和芳族苯胺环的存在平面移开,表明共轭体系的破坏。溶液行为取决于取代基。取决于酰胺侧链取代基的性质,在二酰胺溶液中仅发现酰胺片段和吡啶环的三种相对取向。二酰胺的构象主要取决于取代基的大小,而较少取决于它们的电子性质。研究了二酰胺的溶解度及其相对于and和and离子的金属萃取性能,研究了二酰胺,在二酰胺部分的吡啶环和苯环中带有取代基(一个或两个烷基,烷氧基,氟,羧基) 。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性能。研究了二酰胺的溶解度及其相对于and和and离子的金属萃取性能,研究了二酰胺,在二酰胺部分的吡啶环和苯环中带有取代基(一个或两个烷基,烷氧基,氟,羧基) 。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性质。研究了二酰胺的溶解度及其相对于and和and离子的金属萃取性能,研究了二酰胺,在二酰胺部分的吡啶环和苯环中带有取代基(一个或两个烷基,烷氧基,氟,羧基) 。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性能。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性能。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性质。
更新日期:2018-02-20
中文翻译:

基于2,2'-联吡啶骨架的A提取剂结构的筛选:一种用于N / O2-四齿配体库的简单方法,用于合理设计An / Ln提取剂
合成了一个2,2'-联吡啶-6,6'-二羧酸N-乙基苯胺的文库,以评估侧链对提取性能的影响。各种二酰胺的结构在X射线下以固态研究,在溶液中通过2D NMR研究。在固态状态下,二酰胺具有平坦的Cs几何形状,而与取代基的性质和位置无关。晶体中的羧基从吡啶和芳族苯胺环的存在平面移开,表明共轭体系的破坏。溶液行为取决于取代基。取决于酰胺侧链取代基的性质,在二酰胺溶液中仅发现酰胺片段和吡啶环的三种相对取向。二酰胺的构象主要取决于取代基的大小,而较少取决于它们的电子性质。研究了二酰胺的溶解度及其相对于and和and离子的金属萃取性能,研究了二酰胺,在二酰胺部分的吡啶环和苯环中带有取代基(一个或两个烷基,烷氧基,氟,羧基) 。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性能。研究了二酰胺的溶解度及其相对于and和and离子的金属萃取性能,研究了二酰胺,在二酰胺部分的吡啶环和苯环中带有取代基(一个或两个烷基,烷氧基,氟,羧基) 。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性质。研究了二酰胺的溶解度及其相对于and和and离子的金属萃取性能,研究了二酰胺,在二酰胺部分的吡啶环和苯环中带有取代基(一个或两个烷基,烷氧基,氟,羧基) 。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性能。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性能。吡啶部分中的对称取代严重降低了溶解度和金属萃取。金属离子的回收在很大程度上取决于苯胺部分的取代。分布系数更多地取决于取代基的空间排列,而在较小程度上取决于其电子性质。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号