当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
InCl3‐Assisted Eco‐Friendly Approach for N‐Fused 1,4‐Dihydropyridine Scaffolds via Ring Opening Michael Addition of Cyclic Nitroketene and Iminocoumarin: Synthesis and DFT Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-02-20 , DOI: 10.1002/slct.201702718
Dhanasekar Elumalai 1 , Ramachandran Gnanasekaran 1 , Saraswathi Leelakrishnan 2 , Gunavathy Nachimuthu 2 , Tharanikkarasu Kannan 1 , Thirumalai Perumal Paramasivam 3 , Kamalraja Jayabal 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-02-20 , DOI: 10.1002/slct.201702718
Dhanasekar Elumalai 1 , Ramachandran Gnanasekaran 1 , Saraswathi Leelakrishnan 2 , Gunavathy Nachimuthu 2 , Tharanikkarasu Kannan 1 , Thirumalai Perumal Paramasivam 3 , Kamalraja Jayabal 1
Affiliation
|
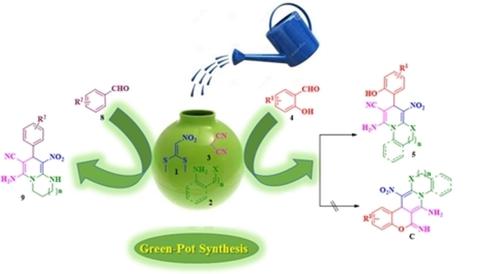
A novel strategy was developed for the synthesis of five different N‐fused 1,4‐dihydropyridine (1,4 DHP) scaffolds such as imidazopyridine, pyridopyrimidine, benzoimidazopyridine, thiazolopyridine and benzopyridooxazine derivatives by coupling nitroketene S,S‐acetal, various nitrogen containing dinucleophiles, malanonitrile and substituted salicylaldehydes/aldehydes in the presence of InCl3 as catalyst in water‐EtOH solvent mixture under reflux condition. Furthermore, mechanism for the formation of 1,4‐DHPs was explored through experimental and DFT calculations. DFT studies reveal that the reaction went through lower energy triheterocyclic intermediate than the higher energy chromenoimidazopyridine imtermediate. The attractive features of this protocol include short reaction time, easy separation of the product without chromatographic purification, simple execution with excellent yield and possibility to synthesize structurally diverse 1,4‐dihydropyridine derivatives through greener approach.
中文翻译:

InCl3辅助的N-融合的1,4-二氢吡啶骨架的生态友好方法,通过环硝基酮和亚氨基香豆素的开环迈克尔加成反应:合成和DFT研究
通过偶联硝基酮S,缩醛和各种含氮化合物,开发了一种新颖的策略来合成五种不同的N-稠合的1,4-二氢吡啶(1,4 DHP)支架,例如咪唑并吡啶,吡啶并嘧啶,苯并咪唑并吡啶,噻唑并吡啶和苯并吡啶并恶嗪衍生物。 InCl 3存在下的双亲核试剂,丙二腈和取代的水杨醛/醛在回流条件下作为水-乙醇溶剂混合物中的催化剂。此外,通过实验和DFT计算探索了1,4-DHP的形成机理。DFT研究表明,该反应通过的是低能级三杂环中间产物,而不是高能级间苯二甲酰亚胺咪唑并吡啶。该方案的吸引人的特征包括反应时间短,无需色谱纯化即可轻松分离产物,易于操作且收率高以及通过更绿色的方法合成结构多样的1,4-二氢吡啶衍生物的可能性。
更新日期:2018-02-20
中文翻译:

InCl3辅助的N-融合的1,4-二氢吡啶骨架的生态友好方法,通过环硝基酮和亚氨基香豆素的开环迈克尔加成反应:合成和DFT研究
通过偶联硝基酮S,缩醛和各种含氮化合物,开发了一种新颖的策略来合成五种不同的N-稠合的1,4-二氢吡啶(1,4 DHP)支架,例如咪唑并吡啶,吡啶并嘧啶,苯并咪唑并吡啶,噻唑并吡啶和苯并吡啶并恶嗪衍生物。 InCl 3存在下的双亲核试剂,丙二腈和取代的水杨醛/醛在回流条件下作为水-乙醇溶剂混合物中的催化剂。此外,通过实验和DFT计算探索了1,4-DHP的形成机理。DFT研究表明,该反应通过的是低能级三杂环中间产物,而不是高能级间苯二甲酰亚胺咪唑并吡啶。该方案的吸引人的特征包括反应时间短,无需色谱纯化即可轻松分离产物,易于操作且收率高以及通过更绿色的方法合成结构多样的1,4-二氢吡啶衍生物的可能性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号