当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction vs Preorganization in Enzyme Catalysis. A Dispute That Calls for Resolution.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-06-19 , DOI: 10.1021/acschembio.8b01029 Fredric M Menger 1 , Faruk Nome 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-06-19 , DOI: 10.1021/acschembio.8b01029 Fredric M Menger 1 , Faruk Nome 2
Affiliation
|
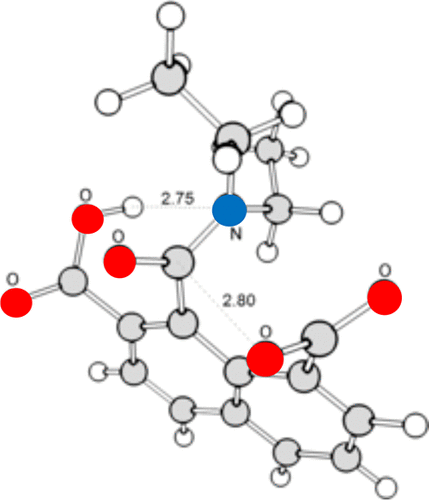
This essay focuses on the debate between Warshel et al. (proponents of preorganization) and Menger and Nome (proponents of spatiotemporal effects) over the source of fast enzyme catalysis. The Warshel model proposes that the main function of enzymes is to push the solvent coordinate toward the transition state. Other physical-organic factors (e.g., desolvation, entropic effects, ground state destabilization, etc.) do not, ostensibly, contribute substantially to the rate. Indeed, physical organic chemistry in its entirety was claimed to be "irrelevant to an enzyme's active site". Preorganization had been applied by Warshel to his "flagship" enzyme, ketosteroid isomerase, but we discuss troubling issues with their ensuing analysis. For example, the concepts of "general acid" and "general base", known to play a role in this enzyme's mechanism, are ignored in the text. In contrast, the spatiotemporal theory postulates that enzyme-like rates (i.e., accelerations >108) occur when two functionalities are held rigidly at contact distances less than ca. 3 Å. Numerous diverse organic systems are shown to bear this out experimentally. Many of these are intramolecular systems where distances between functionalities are known. Among them are fast intramolecular systems where strain is actually generated during the reaction, thereby excluding steric compression as a source of the observed enzyme-like rates. Finally, the account ends with structural data from four active sites of enzymes, obtained by others, all showing contact distances between substrate analogues and enzyme. To our knowledge, contact distances less than the diameter of water are found universally among enzymes, and it is to this fact that we attribute their extremely fast rates given the assumption that enzymes, whatever their particular mechanism, obey elementary chemical principles.
中文翻译:

酶催化中的相互作用与预组织。需要解决的争议。
本文重点讨论Warshel等人之间的争论。(预先组织化的支持者)和Menger和Nome(时空效应的支持者)超越了快速酶催化的来源。Warshel模型提出酶的主要功能是将溶剂坐标推向过渡态。表面上,其他物理有机因素(例如,去溶剂化,熵效应,基态不稳定等)基本上不会对速率产生影响。实际上,据称物理有机化学整体上“与酶的活性位点无关”。Warshel已对他的“旗舰”酶(酮类固醇异构酶)应用了预组织,但是我们在随后的分析中讨论了令人不安的问题。例如,“通用酸”和“通用碱”的概念,已知在这种酶的机制中起作用的物质,在本文中被忽略。相比之下,时空理论假设当两个功能被严格保持在小于ca的接触距离时,会出现类似酶的速率(即加速度> 108)。3Å。实验证明了多种多样的有机体系。其中许多是分子内系统,其中功能之间的距离是已知的。其中有快速分子内系统,其中在反应过程中实际上产生了应变,因此排除了空间压缩作为观察到的酶样速率的来源。最后,该账目以来自其他四个酶的酶活性位点的结构数据结尾,这些数据均显示了底物类似物与酶之间的接触距离。据我们所知,
更新日期:2019-05-31
中文翻译:

酶催化中的相互作用与预组织。需要解决的争议。
本文重点讨论Warshel等人之间的争论。(预先组织化的支持者)和Menger和Nome(时空效应的支持者)超越了快速酶催化的来源。Warshel模型提出酶的主要功能是将溶剂坐标推向过渡态。表面上,其他物理有机因素(例如,去溶剂化,熵效应,基态不稳定等)基本上不会对速率产生影响。实际上,据称物理有机化学整体上“与酶的活性位点无关”。Warshel已对他的“旗舰”酶(酮类固醇异构酶)应用了预组织,但是我们在随后的分析中讨论了令人不安的问题。例如,“通用酸”和“通用碱”的概念,已知在这种酶的机制中起作用的物质,在本文中被忽略。相比之下,时空理论假设当两个功能被严格保持在小于ca的接触距离时,会出现类似酶的速率(即加速度> 108)。3Å。实验证明了多种多样的有机体系。其中许多是分子内系统,其中功能之间的距离是已知的。其中有快速分子内系统,其中在反应过程中实际上产生了应变,因此排除了空间压缩作为观察到的酶样速率的来源。最后,该账目以来自其他四个酶的酶活性位点的结构数据结尾,这些数据均显示了底物类似物与酶之间的接触距离。据我们所知,






























 京公网安备 11010802027423号
京公网安备 11010802027423号