当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Reactivity and Leaching of a Sodium Silicate Glass under an Aqueous Environment: A ReaxFF Molecular Dynamics Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-06-15 , DOI: 10.1021/acs.jpcc.9b02940 Seung Ho Hahn 1 , Adri C. T. van Duin 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-06-15 , DOI: 10.1021/acs.jpcc.9b02940 Seung Ho Hahn 1 , Adri C. T. van Duin 1
Affiliation
|
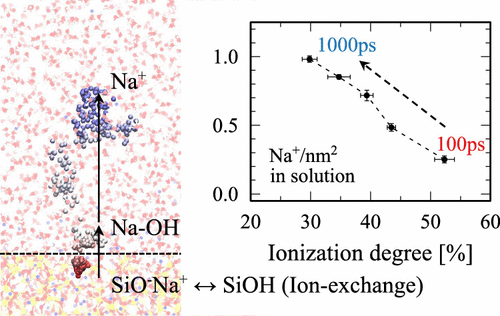
The presence of leachable ions in multicomponent silicate glasses complicates the understanding of their surface structure and chemistry under a humid or aqueous environment, in particular when compared to pure silica–water interactions. Here, we performed large-scale ReaxFF reactive molecular dynamics (MD) simulations and investigated the structural and chemical modifications that take place at the sodium silicate glass–water interface. From our simulation results, we observed that the water interaction gradually replaces sodium ions associated with the surface nonbridging oxygen (NBO) and found that molecular water dissociates into H+ and OH– in the presence of these modifier cations. The protonation of the NBO site produces silanol (SiOH), and our results denote that protons can diffuse further into the bulk region by the discrete proton hopping between two adjacent NBO sites. Consequently, the number of silanol formation increases along the glass–water reaction, rather than reaching an equilibrium point within the nanosecond time scale of our MD simulations. In addition, sodium leaching and its mobility at the interface were studied to provide adequate information on how the ion-exchange process dictates their transport within the interface. Sodium ions are bound close to the surface as Na–OH ion pairs during the initial stages of the water interaction and exhibits a certain residence time at the surface before they are released to the aqueous media. The reactive MD simulation presented here can successfully provide physical insights needed to understand the surface chemistry with the time and length scales which would otherwise be difficult for first-principles or experimental studies to evaluate.
中文翻译:

水性环境下硅酸钠玻璃的表面反应性和浸出:ReaxFF分子动力学研究
多组分硅酸盐玻璃中可浸出离子的存在使在湿润或水性环境下(尤其是与纯硅水相互作用时)对其表面结构和化学性质的理解变得更加复杂。在这里,我们进行了大规模的ReaxFF反应分子动力学(MD)模拟,并研究了在硅酸钠玻璃-水界面发生的结构和化学修饰。从我们的模拟结果中,我们观察到水的相互作用逐渐取代与表面非桥接氧(NBO)相关联的钠离子,发现水分子解离成H +和OH -在这些改性剂阳离子的存在下。NBO位点的质子化产生硅烷醇(SiOH),我们的结果表明,质子可以通过两个相邻NBO位点之间的离散质子跳跃而进一步扩散到主体区域中。因此,硅烷醇形成的数量随着玻璃水反应的增加而增加,而不是在我们的MD模拟的纳秒级时间内达到平衡点。此外,还对钠的浸出及其在界面上的迁移率进行了研究,以提供有关离子交换过程如何决定其在界面内传输的充分信息。在水相互作用的初始阶段,钠离子以Na-OH离子对的形式结合在表面附近,并在释放到水介质之前在表面表现出一定的停留时间。
更新日期:2019-06-16
中文翻译:

水性环境下硅酸钠玻璃的表面反应性和浸出:ReaxFF分子动力学研究
多组分硅酸盐玻璃中可浸出离子的存在使在湿润或水性环境下(尤其是与纯硅水相互作用时)对其表面结构和化学性质的理解变得更加复杂。在这里,我们进行了大规模的ReaxFF反应分子动力学(MD)模拟,并研究了在硅酸钠玻璃-水界面发生的结构和化学修饰。从我们的模拟结果中,我们观察到水的相互作用逐渐取代与表面非桥接氧(NBO)相关联的钠离子,发现水分子解离成H +和OH -在这些改性剂阳离子的存在下。NBO位点的质子化产生硅烷醇(SiOH),我们的结果表明,质子可以通过两个相邻NBO位点之间的离散质子跳跃而进一步扩散到主体区域中。因此,硅烷醇形成的数量随着玻璃水反应的增加而增加,而不是在我们的MD模拟的纳秒级时间内达到平衡点。此外,还对钠的浸出及其在界面上的迁移率进行了研究,以提供有关离子交换过程如何决定其在界面内传输的充分信息。在水相互作用的初始阶段,钠离子以Na-OH离子对的形式结合在表面附近,并在释放到水介质之前在表面表现出一定的停留时间。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号