Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2019-05-31 , DOI: 10.1016/j.apcatb.2019.117796 Fengxia Deng , Sixing Li , Minghua Zhou , Yingshi Zhu , Shan Qiu , Kehong Li , Fang Ma , Jizhou Jiang
|
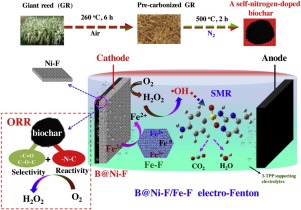
A nickel-foam cathode modified by a self-nitrogen-doped biochar derived from waste giant reed was synthesized. The fabricated cathode ([email protected]) proved to be with high oxygen reaction reactive (ORR) reactivity and H2O2 selectivity (70.41%) owing to the enrichment of oxygen functional groups and pyridinic N when low-temperature pyrolyzed biochar was incorporated. The charge transfer resistance of [email protected] decreased to 7.18 Ω, which was 95.7 Ω for the original nickel-foam, proving by electrochemical impedance spectroscopy (EIS). Expectedly, Its H2O2 accumulation improved 14 times, thus making it comparable with commonly used electrodes like carbon cloth and graphite plate. Subsequently, [email protected] cathode and iron-foam (Fe-F) catalyst were firstly used in the electro-Fenton (EF) process for sulfamerazine (SMR) degradation. Double-functional polyphosphate electrolytes including tetrapolyphosphate (4-TPP), tripolyphosphate (3-TPP), pyrophosphate (PP) and Na3PO4 were compared with the conventional Na2SO4 electrolyte in EF for SMR degradation. The absolute rate constant for oxidation of SMR by OH was determined to be (3.4 ± 0.09) × 109 M−1 s−1. SMR degradation enhancement in the presence of polyphosphate-based electrolytes is associated with bulk
OH generation from Fe2+- polyphosphate ligand complexes via O2 activation. The Fe2+-3-TPP complexes have relatively higher oxidation ability compared to Fe2+-PP, Fe2+-PO4 species. A plausible SMR oxidation pathway is proposed based on the by-products detected by UPLC-MS/MS and density functional theory (DFT) calculations. The dominant SMR degradation pathway was hydroxylation of aniline residue of SMR, followed with the cleavage of
S
N
and then breakage of aromatic rings.
中文翻译:

电芬顿中具有铁泡沫催化剂的生物炭改性镍泡沫阴极,用于磺胺嘧啶的降解
合成了由废巨型芦苇衍生的自氮掺杂生物炭修饰的镍泡沫阴极。掺入低温热解生物炭后,由于氧官能团和吡啶二价氮的富集,制成的阴极([受电子邮件保护])具有高的氧反应活性(ORR)反应性和H 2 O 2选择性(70.41%)。 。通过电化学阻抗谱(EIS)证明,[电子邮件保护的]电荷转移电阻降低至7.18Ω,原始镍泡沫的电荷转移电阻为95.7Ω。预期其H 2 O 2堆积提高了14倍,因此可与碳布和石墨板等常用电极媲美。随后,首先在电芬顿(EF)工艺中使用[电子邮件保护的]阴极和铁泡沫(Fe-F)催化剂来降解磺胺嘧啶(SMR)。将包括四聚磷酸盐(4-TPP),三聚磷酸盐(3-TPP),焦磷酸盐(PP)和Na 3 PO 4的双功能聚磷酸盐电解质与EF中常规的Na 2 SO 4电解质进行SMR降解。通过OH氧化SMR的绝对速率常数确定为(3.4±0.09)×10 9 M -1 s -1。在基于多磷酸盐的电解质存在下,SMR降解的增强与
通过Fe 2 + -多磷酸盐配体配合物通过O 2活化而生成大量OH有关。与Fe 2+ -PP,Fe 2+ -PO 4物种相比,Fe 2+ -3-TPP配合物具有相对较高的氧化能力。基于UPLC-MS / MS检测到的副产物和密度泛函理论(DFT)计算,提出了一种可行的SMR氧化途径。SMR的主要降解途径是SMR的苯胺残基羟基化,然后S N裂解,然后芳环断裂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号