当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion exchange collaborating coordination substitution: More efficient Cr(VI) removal performance of a water-stable CuII-MOF material.
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2019-05-31 , DOI: 10.1016/j.jhazmat.2019.05.112 Zhichao Shao 1 , Chao Huang 2 , Qiong Wu 1 , Yujie Zhao 1 , Wenjuan Xu 1 , Yeye Liu 1 , Jian Dang 1 , Hongwei Hou 1
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2019-05-31 , DOI: 10.1016/j.jhazmat.2019.05.112 Zhichao Shao 1 , Chao Huang 2 , Qiong Wu 1 , Yujie Zhao 1 , Wenjuan Xu 1 , Yeye Liu 1 , Jian Dang 1 , Hongwei Hou 1
Affiliation
|
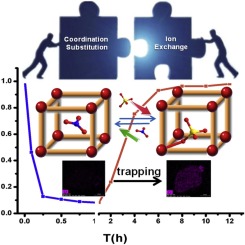
An unusual water-stable cationic metal-organic framework {[Cu(L)0.5(bpe)(H2O)](NO3)•(H2O)0.5}n (1) (H4L = bis(3,5-dicarboxypyridinium)-p-xylylene) was synthesized, which was developed into an effective capture material for removal chromate from water. The results show that this material efficiently traps HCrO4- pollutant ions via single-crystal to single-crystal (SCSC) coordination substitution process. The HCrO4- uptake capacity of 1 is high to 190 mg/g. Meaningfully, the structure of 1-HCrO4 ({[Cu(L)0.5(bpe)(HCrO4)]}n) can be accurately obtained by single-crystal X-ray diffraction, where the chromate enter the framework to form stable coordination with central metal ions Cu2+. This is the first example of a stable coordination between chromate and the framework during the capture process. The captured HCrO4- are not dissociated easily into the solution due to the coordination bond. This interaction makes the enrichment of HCrO4- more stable and the capture capacity excellent. Furthermore, the HCrO4- releasing process displays good regeneration in a single crystal state, which further elaborates the reversible SCSC transformation. The mechanism of Cr(VI) removal was also confirmed by DFT calculation studies. This work provides a new way to design and develop efficient MOF capture materials.
中文翻译:

离子交换协作配位取代:水溶性CuII-MOF材料的更有效的Cr(VI)去除性能。
不寻常的水稳定性阳离子金属有机骨架{[Cu(L)0.5(bpe)(H2O)](NO3)•(H2O)0.5} n(1)(H4L =双(3,5-二羧基吡啶鎓)-p合成二甲苯),将其发展成为一种有效的捕集材料,用于从水中去除铬酸盐。结果表明,该材料通过单晶至单晶(SCSC)配位取代过程有效捕获了HCrO4-污染物离子。1的HCrO4-吸收能力高达190 mg / g。意思是,可以通过单晶X射线衍射准确地获得1-HCrO4({[Cu(L)0.5(bpe)(HCrO4)]} n)的结构,其中铬酸盐进入骨架以形成稳定的配位基。中心金属离子Cu2 +。这是捕获过程中铬酸盐与框架之间稳定协调的第一个例子。由于配位键,捕获的HCrO4-很难分解成溶液。这种相互作用使HCrO4-的富集更加稳定,捕获能力极佳。此外,释放HCrO4的过程在单晶状态下显示出良好的再生,这进一步阐明了可逆的SCSC转化。DFT计算研究也证实了Cr(VI)去除的机理。这项工作为设计和开发有效的MOF捕获材料提供了一种新方法。DFT计算研究也证实了Cr(VI)去除的机理。这项工作为设计和开发有效的MOF捕获材料提供了一种新方法。DFT计算研究也证实了Cr(VI)去除的机理。这项工作为设计和开发有效的MOF捕获材料提供了一种新方法。
更新日期:2019-05-31
中文翻译:

离子交换协作配位取代:水溶性CuII-MOF材料的更有效的Cr(VI)去除性能。
不寻常的水稳定性阳离子金属有机骨架{[Cu(L)0.5(bpe)(H2O)](NO3)•(H2O)0.5} n(1)(H4L =双(3,5-二羧基吡啶鎓)-p合成二甲苯),将其发展成为一种有效的捕集材料,用于从水中去除铬酸盐。结果表明,该材料通过单晶至单晶(SCSC)配位取代过程有效捕获了HCrO4-污染物离子。1的HCrO4-吸收能力高达190 mg / g。意思是,可以通过单晶X射线衍射准确地获得1-HCrO4({[Cu(L)0.5(bpe)(HCrO4)]} n)的结构,其中铬酸盐进入骨架以形成稳定的配位基。中心金属离子Cu2 +。这是捕获过程中铬酸盐与框架之间稳定协调的第一个例子。由于配位键,捕获的HCrO4-很难分解成溶液。这种相互作用使HCrO4-的富集更加稳定,捕获能力极佳。此外,释放HCrO4的过程在单晶状态下显示出良好的再生,这进一步阐明了可逆的SCSC转化。DFT计算研究也证实了Cr(VI)去除的机理。这项工作为设计和开发有效的MOF捕获材料提供了一种新方法。DFT计算研究也证实了Cr(VI)去除的机理。这项工作为设计和开发有效的MOF捕获材料提供了一种新方法。DFT计算研究也证实了Cr(VI)去除的机理。这项工作为设计和开发有效的MOF捕获材料提供了一种新方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号