Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile and Versatile Sol–Gel Strategy for the Preparation of a High-Loaded ZnO/SiO2 Adsorbent for Room-Temperature H2S Removal
Langmuir ( IF 3.7 ) Pub Date : 2019-05-29 00:00:00 , DOI: 10.1021/acs.langmuir.9b00853 Chao Yang , Jiawei Kou , Huiling Fan , Zhen Tian 1 , Wei Kong , Ju Shangguan
Langmuir ( IF 3.7 ) Pub Date : 2019-05-29 00:00:00 , DOI: 10.1021/acs.langmuir.9b00853 Chao Yang , Jiawei Kou , Huiling Fan , Zhen Tian 1 , Wei Kong , Ju Shangguan
Affiliation
|
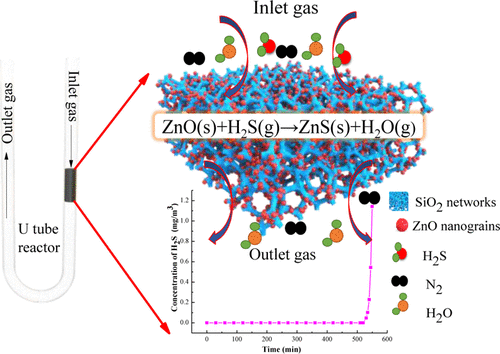
Preparation of well-dispersed ZnO nanograins is necessary to improve their reactivity toward room-temperature H2S removal. However, the challenge to design such a ZnO-based adsorbent with high ZnO loading is yet to be fulfilled. Herein, a facile sol–gel strategy is reported for the preparation of ZnO/SiO2 adsorbents for efficient H2S removal, by innovating a gel-drying method and simultaneously controlling ZnO grain formation through optimizing the molar ratio of ethylene glycol (EG)/nitrates in its precursors. The fabricated adsorbent embedded well-dispersed ZnO nanograins, of approximately 10–15 nm, into a SiO2 matrix (57 wt % ZnO loading) and thus yielded a high H2S removal capacity of 108.9 mg S/g sorbent. Therein, EG was used as a modifier for inhibiting the formation of a denser SiO2 network during the gel drying process and was used as a fuel for promoting the decomposition of nitrates and increasing the surface area of the composites in the subsequent calcination. Modulating the molar ratio of EG/nitrates ≤ 2 in precursors or traditional drying of the gel in an oven should be avoided because these would lead to the oxidation of EG by metallic nitrates and form carboxylate complexes during the gel-drying process. Although the produced ZnO grains had a very small size of less than 5 nm, a layer of monodentate ZnCO3 impurity was formed on the ZnO surface, which will drastically decrease the reactivity of ZnO toward H2S. According to the encouraging results from CuO and Co3O4, this strategy has proved to be versatile for the preparation of other metal oxide/SiO2 adsorbents.
中文翻译:

制备用于室温H 2 S脱除的高负载ZnO / SiO 2吸附剂的简便且多功能的Sol-Gel策略
制备分散良好的ZnO纳米颗粒对于提高其对室温H 2 S去除的反应性是必要的。然而,设计具有高ZnO负载量的基于ZnO的吸附剂的挑战尚待解决。本文中,通过创新的凝胶干燥方法并通过优化乙二醇(EG)的摩尔比来控制ZnO晶粒的形成,据报导了一种用于高效去除H 2 S的ZnO / SiO 2吸附剂的简便溶胶-凝胶策略。/硝酸盐在其前体。制备的吸附剂将约10–15 nm的分散良好的ZnO纳米颗粒嵌入SiO 2基质(ZnO含量为57 wt%)中,从而产生高H 2S去除能力为108.9 mg S / g吸附剂。其中,EG被用作在凝胶干燥过程中抑制形成更致密的SiO 2网络的改性剂,并且被用作在随后的煅烧中促进硝酸盐的分解并增加复合材料的表面积的燃料。应避免调节前体中EG /硝酸盐的摩尔比≤2或在烤箱中传统干燥凝胶,因为这会导致EG在金属干燥过程中被金属硝酸盐氧化并形成羧酸盐配合物。尽管产生的ZnO晶粒的尺寸非常小,小于5 nm,但在ZnO表面形成了单齿ZnCO 3杂质层,这将大大降低ZnO对H 2的反应性S.根据来自CuO和Co 3 O 4的令人鼓舞的结果,该策略已被证明可用于制备其他金属氧化物/ SiO 2吸附剂。
更新日期:2019-05-29
中文翻译:

制备用于室温H 2 S脱除的高负载ZnO / SiO 2吸附剂的简便且多功能的Sol-Gel策略
制备分散良好的ZnO纳米颗粒对于提高其对室温H 2 S去除的反应性是必要的。然而,设计具有高ZnO负载量的基于ZnO的吸附剂的挑战尚待解决。本文中,通过创新的凝胶干燥方法并通过优化乙二醇(EG)的摩尔比来控制ZnO晶粒的形成,据报导了一种用于高效去除H 2 S的ZnO / SiO 2吸附剂的简便溶胶-凝胶策略。/硝酸盐在其前体。制备的吸附剂将约10–15 nm的分散良好的ZnO纳米颗粒嵌入SiO 2基质(ZnO含量为57 wt%)中,从而产生高H 2S去除能力为108.9 mg S / g吸附剂。其中,EG被用作在凝胶干燥过程中抑制形成更致密的SiO 2网络的改性剂,并且被用作在随后的煅烧中促进硝酸盐的分解并增加复合材料的表面积的燃料。应避免调节前体中EG /硝酸盐的摩尔比≤2或在烤箱中传统干燥凝胶,因为这会导致EG在金属干燥过程中被金属硝酸盐氧化并形成羧酸盐配合物。尽管产生的ZnO晶粒的尺寸非常小,小于5 nm,但在ZnO表面形成了单齿ZnCO 3杂质层,这将大大降低ZnO对H 2的反应性S.根据来自CuO和Co 3 O 4的令人鼓舞的结果,该策略已被证明可用于制备其他金属氧化物/ SiO 2吸附剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号