当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Aryl Pyrrolidines as Novel Orally Efficacious Antimalarial Agents. Part 2: 2-Aryl-N-(4-arylpyrrolidin-3-yl)acetamides.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-05-28 00:00:00 , DOI: 10.1021/acsmedchemlett.9b00123 Marvin J Meyers 1 , Jianguang Liu 2 , Zhijun Liu 2 , Hongwei Ma 2 , Linglin Dai 3 , Dickson Adah 3, 4 , Siting Zhao 3 , Xiaofen Li 3 , Xiaorong Liu 2 , Yongzhi Lu 2 , Yanhui Huang 2 , Zhengchao Tu 2 , Xiaoping Chen 3 , Micky D Tortorella 2, 5
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-05-28 00:00:00 , DOI: 10.1021/acsmedchemlett.9b00123 Marvin J Meyers 1 , Jianguang Liu 2 , Zhijun Liu 2 , Hongwei Ma 2 , Linglin Dai 3 , Dickson Adah 3, 4 , Siting Zhao 3 , Xiaofen Li 3 , Xiaorong Liu 2 , Yongzhi Lu 2 , Yanhui Huang 2 , Zhengchao Tu 2 , Xiaoping Chen 3 , Micky D Tortorella 2, 5
Affiliation
|
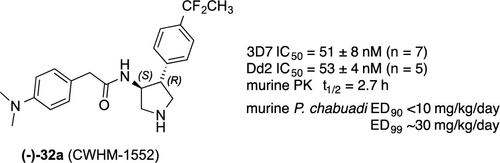
Malaria is caused by infection from the Plasmodium parasite and kills hundreds of thousands of people every year. Emergence of new drug resistant strains of Plasmodium demands identification of new drugs with novel chemotypes and mechanisms of action. As a follow up to our evaluation of 4-aryl-N-benzylpyrrolidine-3-carboxamides as novel pyrrolidine-based antimalarial agents, we describe herein the structure–activity relationships of the reversed amide homologues 2-aryl-N-(4-arylpyrrolidin-3-yl)acetamides. Unlike their carboxamide homologues, acetamide pyrrolidines do not require a third chiral center to be potent inhibitors of P. falciparum and have good pharmacokinetic properties and improved oral efficacy in a mouse model of malaria. Compound (−)-32a (CWHM-1552) has an in vitro IC50 of 51 nM in the P. falciparum 3D7 assay and an in vivo ED90 of <10 mg/kg/day and ED99 of 30 mg/kg/day in a murine P. chabaudi model. Remarkably, the absolute stereochemical preference for this acetamide series (3S,4R) is opposite of that determined for the homologous carboxamide series. Lead compounds for this class have modest affinities for the hERG channel and inhibit CYP 3A4. Additional optimization is needed in order to eliminate these undesired properties from this otherwise promising series of antimalarial compounds.
中文翻译:

4-芳基吡咯烷类作为新型口服有效抗疟药。第2部分:2-芳基-N-(4-芳基吡咯烷-3-基)乙酰胺。
疟疾是由疟原虫寄生虫感染引起的,每年造成数十万人死亡。疟原虫的新抗药性菌株的出现要求鉴定具有新化学型和作用机理的新药物。作为我们对4-芳基-N-苄基吡咯烷-3-羧酰胺作为新型吡咯烷基抗疟剂的评估的后续措施,我们在此描述了反向酰胺同系物2-芳基-N-(4-芳基吡咯烷酮)的结构-活性关系-3-基)乙酰胺。不同于其酰胺酰胺同系物,乙酰胺吡咯烷不需要第三手性中心即可成为P的有效抑制剂。恶性疟并且在疟疾的小鼠模型中具有良好的药代动力学特性和改善的口服功效。化合物(-)-32a(CWHM-1552)在恶性疟原虫3D7分析中的体外IC 50为51 nM ,体内ED 90 <10 mg / kg / day和ED 99为30 mg / kg /鼠P. chabaudi模型中的一天。值得注意的是,此乙酰胺系列(3 S,4 R)与同源羧酰胺系列测定的结果相反。此类先导化合物对hERG通道具有适度的亲和力,并抑制CYP 3A4。为了从这些本来很有希望的抗疟化合物系列中消除这些不良特性,还需要进行其他优化。
更新日期:2019-05-28
中文翻译:

4-芳基吡咯烷类作为新型口服有效抗疟药。第2部分:2-芳基-N-(4-芳基吡咯烷-3-基)乙酰胺。
疟疾是由疟原虫寄生虫感染引起的,每年造成数十万人死亡。疟原虫的新抗药性菌株的出现要求鉴定具有新化学型和作用机理的新药物。作为我们对4-芳基-N-苄基吡咯烷-3-羧酰胺作为新型吡咯烷基抗疟剂的评估的后续措施,我们在此描述了反向酰胺同系物2-芳基-N-(4-芳基吡咯烷酮)的结构-活性关系-3-基)乙酰胺。不同于其酰胺酰胺同系物,乙酰胺吡咯烷不需要第三手性中心即可成为P的有效抑制剂。恶性疟并且在疟疾的小鼠模型中具有良好的药代动力学特性和改善的口服功效。化合物(-)-32a(CWHM-1552)在恶性疟原虫3D7分析中的体外IC 50为51 nM ,体内ED 90 <10 mg / kg / day和ED 99为30 mg / kg /鼠P. chabaudi模型中的一天。值得注意的是,此乙酰胺系列(3 S,4 R)与同源羧酰胺系列测定的结果相反。此类先导化合物对hERG通道具有适度的亲和力,并抑制CYP 3A4。为了从这些本来很有希望的抗疟化合物系列中消除这些不良特性,还需要进行其他优化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号