当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Controlled Organobase-Catalyzed Ring-Opening Polymerization of Morpholine-2,5-Dione Derivatives and Monomer Recovery by Acid-Catalyzed Degradation of the Polymers
Macromolecules ( IF 5.1 ) Pub Date : 2019-05-29 00:00:00 , DOI: 10.1021/acs.macromol.8b02498 Chang-Xia Shi 1 , Yu-Ting Guo 1 , Yu-Huan Wu 1 , Zhao-Yue Li 1 , Yao-Zong Wang 1 , Fu-Sheng Du 1 , Zi-Chen Li 1
Macromolecules ( IF 5.1 ) Pub Date : 2019-05-29 00:00:00 , DOI: 10.1021/acs.macromol.8b02498 Chang-Xia Shi 1 , Yu-Ting Guo 1 , Yu-Huan Wu 1 , Zhao-Yue Li 1 , Yao-Zong Wang 1 , Fu-Sheng Du 1 , Zi-Chen Li 1
Affiliation
|
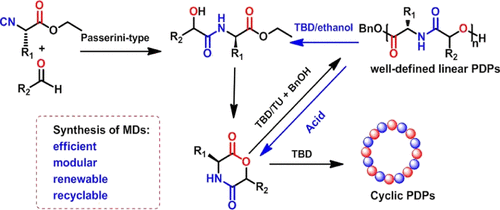
Polydepsipeptides (PDPs) are strictly alternating copolymers of α-hydroxy acids and α-amino acids produced via the ring-opening polymerization (ROP) of morpholino-2,5-dione derivatives (MDs). They have been used as promising biomaterials for their combined high thermal stability and good mechanical properties of polyamides as well as the inherent degradability of polyesters. ROP of MDs is usually carried out at high temperatures with metal catalysts or enzymes, with less control over the polymer molecular weights and dispersities. In this work, we developed a simple and efficient synthetic strategy of a new platform MD via the Passerini-type reaction between an isocyano derivative of the amino acid and an aldehyde, followed by intramolecular esterification. Nine new MDs were synthesized by using this method, and the organobase-catalyzed ROP of these MDs was investigated. When the ROPs of these MDs were catalyzed by either triazabicyclo[4.4.0]dec-5-ene (TBD) or diazabicyclo[5.4.0]undec-7-ene (DBU) in the presence of benzyl alcohol as an initiator, the polymerizations were uncontrolled with the formation of both linear PDPs and cyclic PDPs. By using binary catalytic systems of 1-(3,5-bis(trifluoromethyl)-phenyl-3-cyclohexyl-2-thiourea) (TU) with DBU or TBD ([TU]/[TBD] or [DBU] > 3), the polymerizations became well-controlled, allowing the synthesis of PDPs with controlled molecular weights, low dispersities, as well as block copolymers. Furthermore, cyclic PDPs were obtained when the ROP of these MDs was catalyzed with TBD in the absence of both TU and an initiator. Finally, we used two methods to recover the monomer precursors or pure MD monomers: the TBD-catalyzed alcoholysis of PDPs was very fast and generated the monomer precursors quantitatively, while the acid-catalyzed depolymerization of PDPs led to pure and quantitative monomer recovery.
中文翻译:

吗啉-2,5-二酮衍生物的合成及受控有机碱催化的开环聚合反应及通过酸催化降解聚合物的单体回收率
聚二肽(PDP)是通过吗啉代2,5-二酮衍生物(MD)的开环聚合(ROP)生成的α-羟基酸和α-氨基酸的严格交替共聚物。它们具有很高的热稳定性,良好的聚酰胺机械性能以及聚酯固有的降解性,因此被用作有前途的生物材料。MD的ROP通常在高温下用金属催化剂或酶进行,对聚合物分子量和分散度的控制较少。在这项工作中,我们通过氨基酸异氰酸酯衍生物与醛之间的Passerini型反应,然后进行分子内酯化,开发了一种简单有效的新平台MD合成策略。使用这种方法合成了九个新的MD,并研究了这些MD的有机碱催化的ROP。当在苄醇作为引发剂的存在下,三氮杂双环[4.4.0] dec-5-ene(TBD)或二氮杂双环[5.4.0] undec-7-ene(DBU)催化这些MD的ROP时,线性PDP和环状PDP的形成均无法控制聚合。通过使用1-(3,5-双(三氟甲基)-苯基-3-环己基-2-硫脲)(TU)与DBU或TBD([TU] / [TBD]或[DBU]> 3)的二元催化体系之后,聚合反应得到了很好的控制,从而可以合成分子量受控,分散度低的PDP以及嵌段共聚物。此外,当在不存在TU和引发剂的情况下用TBD催化这些MD的ROP时,获得了环状PDP。最后,我们使用了两种方法来回收单体前体或纯MD单体:
更新日期:2019-05-29
中文翻译:

吗啉-2,5-二酮衍生物的合成及受控有机碱催化的开环聚合反应及通过酸催化降解聚合物的单体回收率
聚二肽(PDP)是通过吗啉代2,5-二酮衍生物(MD)的开环聚合(ROP)生成的α-羟基酸和α-氨基酸的严格交替共聚物。它们具有很高的热稳定性,良好的聚酰胺机械性能以及聚酯固有的降解性,因此被用作有前途的生物材料。MD的ROP通常在高温下用金属催化剂或酶进行,对聚合物分子量和分散度的控制较少。在这项工作中,我们通过氨基酸异氰酸酯衍生物与醛之间的Passerini型反应,然后进行分子内酯化,开发了一种简单有效的新平台MD合成策略。使用这种方法合成了九个新的MD,并研究了这些MD的有机碱催化的ROP。当在苄醇作为引发剂的存在下,三氮杂双环[4.4.0] dec-5-ene(TBD)或二氮杂双环[5.4.0] undec-7-ene(DBU)催化这些MD的ROP时,线性PDP和环状PDP的形成均无法控制聚合。通过使用1-(3,5-双(三氟甲基)-苯基-3-环己基-2-硫脲)(TU)与DBU或TBD([TU] / [TBD]或[DBU]> 3)的二元催化体系之后,聚合反应得到了很好的控制,从而可以合成分子量受控,分散度低的PDP以及嵌段共聚物。此外,当在不存在TU和引发剂的情况下用TBD催化这些MD的ROP时,获得了环状PDP。最后,我们使用了两种方法来回收单体前体或纯MD单体:






























 京公网安备 11010802027423号
京公网安备 11010802027423号