Nature Communications ( IF 14.7 ) Pub Date : 2019-05-29 , DOI: 10.1038/s41467-019-10220-1 Stephen A Marshall 1 , Karl A P Payne 1 , Karl Fisher 1 , Mark D White 1, 2 , Aisling Ní Cheallaigh 1, 3 , Arune Balaikaite 1 , Stephen E J Rigby 1 , David Leys 1
|
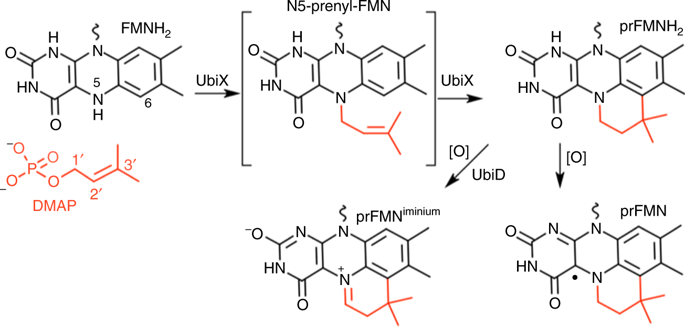
The UbiX-UbiD enzymes are widespread in microbes, acting in concert to decarboxylate alpha-beta unsaturated carboxylic acids using a highly modified flavin cofactor, prenylated FMN (prFMN). UbiX serves as the flavin prenyltransferase, extending the isoalloxazine ring system with a fourth non-aromatic ring, derived from sequential linkage between a dimethylallyl moiety and the FMN N5 and C6. Using structure determination and solution studies of both dimethylallyl monophosphate (DMAP) and dimethyallyl pyrophosphate (DMAPP) dependent UbiX enzymes, we reveal the first step, N5-C1’ bond formation, is contingent on the presence of a dimethylallyl substrate moiety. Hence, an SN1 mechanism similar to other prenyltransferases is proposed. Selected variants of the (pyro)phosphate binding site are unable to catalyse subsequent Friedel-Crafts alkylation of the flavin C6, but can be rescued by addition of (pyro)phosphate. Thus, retention of the (pyro)phosphate leaving group is required for C6-C3’ bond formation, resembling pyrophosphate initiated class I terpene cyclase reaction chemistry.
中文翻译:

UbiX黄素异戊二烯基转移酶的反应机制类似于I类萜环化酶。
UbiX-UbiD酶广泛存在于微生物中,它们共同作用是使用高度修饰的黄素辅因子,戊烯基化的FMN(prFMN)使α-β不饱和羧酸脱羧。UbiX用作黄素异戊二烯基转移酶,扩展了异四恶嗪环系统的第四个非芳族环,该环衍生自二甲基烯丙基部分与FMN N5和C6之间的顺序连接。使用结构确定和对二甲基烯丙基单磷酸酯(DMAP)和焦磷酸二甲基烯丙基焦糖(DMAPP)依赖的UbiX酶的溶液研究,我们揭示了第一步,N5-C1'键的形成取决于二甲基烯丙基底物部分的存在。因此,S N提出了一种类似于其他异戊二烯基转移酶的机制。(焦)磷酸结合位点的选定变体不能催化黄素C6的随后的弗瑞德-克来福特烷基化,但是可以通过添加(焦)磷酸来挽救。因此,保留(焦)磷酸离去基团对于C6-C3'键的形成是必需的,类似于焦磷酸引发的I类萜烯环化酶反应化学。

































 京公网安备 11010802027423号
京公网安备 11010802027423号