当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of racemic 2-(aminomethyl)cyclopropane-1,1-dicarboxylic acid as a new constrained γ-amino dicarboxylic acid bypassing alkyl 3-aza-2-oxobicyclo[3.1.0]hexane-1-carboxylates
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-06-13 , DOI: 10.1002/ejoc.201900542 Sven Mangelinckx 1 , Marina Kostić 2 , Simon Backx 1 , Biljana Petrović 2 , Norbert De Kimpe 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-06-13 , DOI: 10.1002/ejoc.201900542 Sven Mangelinckx 1 , Marina Kostić 2 , Simon Backx 1 , Biljana Petrović 2 , Norbert De Kimpe 1
Affiliation
|
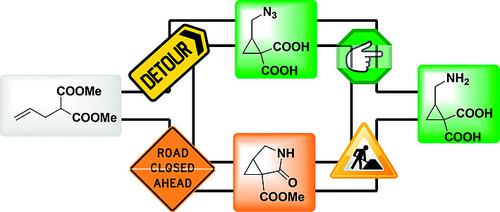
The first synthesis of racemic 2-(aminomethyl)cyclopropane-1,1-dicarboxylic acid was developed involving sequential iodocarbocyclization, azidation, saponification and reduction of dimethyl 2-allylmalonate. The developed synthetic pathway avoids reactions such as ring opening of the cyclopropane ring toward acyclic delta-amino carboxylic acid derivatives or lactamisation toward bicyclic methyl 3-aza-2-oxobicyclo[3.1.0]hexane-1-carboxylates which occur in alternative synthetic strategies.
中文翻译:

外消旋 2-(氨基甲基) 环丙烷-1,1-二羧酸作为一种新的约束 γ-氨基二羧酸绕过 3-氮杂-2-氧代双环 [3.1.0] 己烷-1-羧酸烷基酯的合成
外消旋 2-(氨基甲基) 环丙烷-1,1-二羧酸的首次合成涉及顺序碘碳环化、叠氮化、皂化和 2-烯丙丙二酸二甲酯的还原。开发的合成途径避免了诸如环丙烷环向无环 δ-氨基羧酸衍生物开环或向双环甲基 3-氮杂-2-氧代双环 [3.1.0] 己烷-1-羧酸酯的内酰胺化等反应,这些反应发生在替代合成策略中.
更新日期:2019-06-13
中文翻译:

外消旋 2-(氨基甲基) 环丙烷-1,1-二羧酸作为一种新的约束 γ-氨基二羧酸绕过 3-氮杂-2-氧代双环 [3.1.0] 己烷-1-羧酸烷基酯的合成
外消旋 2-(氨基甲基) 环丙烷-1,1-二羧酸的首次合成涉及顺序碘碳环化、叠氮化、皂化和 2-烯丙丙二酸二甲酯的还原。开发的合成途径避免了诸如环丙烷环向无环 δ-氨基羧酸衍生物开环或向双环甲基 3-氮杂-2-氧代双环 [3.1.0] 己烷-1-羧酸酯的内酰胺化等反应,这些反应发生在替代合成策略中.































 京公网安备 11010802027423号
京公网安备 11010802027423号