当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mesoporous gold nanospheres via thiolate–Au(i) intermediates†
Chemical Science ( IF 7.6 ) Pub Date : 2019-05-28 00:00:00 , DOI: 10.1039/c9sc01728c Hao Lv 1, 2, 3, 4, 5 , Dongdong Xu 1, 2, 3, 4, 5 , Joel Henzie 6, 7, 8, 9, 10 , Ji Feng 11, 12, 13, 14 , Aaron Lopes 14, 15, 16, 17 , Yusuke Yamauchi 6, 7, 8, 9, 10 , Ben Liu 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2019-05-28 00:00:00 , DOI: 10.1039/c9sc01728c Hao Lv 1, 2, 3, 4, 5 , Dongdong Xu 1, 2, 3, 4, 5 , Joel Henzie 6, 7, 8, 9, 10 , Ji Feng 11, 12, 13, 14 , Aaron Lopes 14, 15, 16, 17 , Yusuke Yamauchi 6, 7, 8, 9, 10 , Ben Liu 1, 2, 3, 4, 5
Affiliation
|
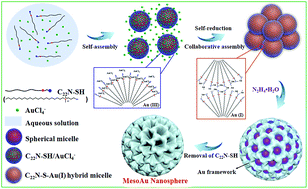
Mesoporous gold (mesoAu) nanospheres support enhanced (electro)catalytic performance owing to their three-dimensional (3D) interior mesochannels that expose abundant active sites and facilitate electron/mass transfers. Although various porous Nanostructured Au has been fabricated by electrochemical reduction, alloying–dealloying and hard/soft templating methods, successful synthesis of mesoAu nanospheres with tailorable sizes and porosities remains a big challenge. Here we describe a novel surfactant-directed synthetic route to fabricate mesoAu nanospheres with 3D interconnected mesochannels by using the amphiphilic surfactant of C22H45N+(CH3)2–C3H6–SH (Cl−) (C22N–SH) as the mesopore directing agent. C22N–SH can not only self-reduce trivalent Au(III)Cl4− to monovalent Au(I), but also form polymeric C22N–S–Au(I) intermediates via covalent bonds. These C22N–S–Au(I) intermediates facilitate the self-assembly into spherical micelles and inhibit the mobility of Au precursors, enabling the crystallization nucleation and growth of the mesoAu nanospheres via in situ chemical reduction. The synthetic strategy can be further extended to tailor the sizes/porosities and surface optical properties of the mesoAu nanospheres. The mesoAu nanospheres exhibit remarkably enhanced mass/specific activity and improved stability in methanol electrooxidation, demonstrating far better performance than non-porous Au nanoparticles and previously reported Au nanocatalysts. The synthetic route differs markedly from other long-established soft-templating approaches, providing a new avenue to grow metal nanocrystals with desirable nanostructures and functions.
中文翻译:

通过硫醇盐–Au(i)中间体的中 孔金纳米球†
介孔金(mesoAu)纳米球由于其三维(3D)内部介观通道而暴露出丰富的活性位点并促进电子/质量转移,因此具有增强的(电)催化性能。尽管已经通过电化学还原,合金化-脱合金以及硬/软模板方法制备了各种多孔纳米结构金,但成功合成具有可定制尺寸和孔隙率的mesoAu纳米球仍然是一个巨大的挑战。在这里,我们描述了一种新型的表面活性剂定向合成路线,以通过使用C 22 H 45 N +(CH 3)2 –C 3 H 6 –SH(Cl−)(C 22 N–SH)作为中孔导向剂。Ç 22 N-SH不仅可以自三价降低的Au( III)氯4 -单价金(我),但也形成聚合Ç 22 N-S-AU(我)的中间体通过共价键。这些C 22 N–S–Au( I)中间体有助于自组装成球形胶束并抑制Au前体的迁移,从而通过原位形成mesoAu纳米球的结晶成核和生长化学还原。合成策略可以进一步扩展以定制mesoAu纳米球的尺寸/孔隙率和表面光学性质。mesoAu纳米球在甲醇电氧化中表现出显着增强的质量/比活性和改善的稳定性,证明其性能远远优于无孔的Au纳米颗粒和先前报道的Au纳米催化剂。合成路线与其他长期建立的软模板方法截然不同,这为生长具有所需纳米结构和功能的金属纳米晶体提供了一条新途径。
更新日期:2019-05-28
中文翻译:

通过硫醇盐–Au(i)中间体的中 孔金纳米球†
介孔金(mesoAu)纳米球由于其三维(3D)内部介观通道而暴露出丰富的活性位点并促进电子/质量转移,因此具有增强的(电)催化性能。尽管已经通过电化学还原,合金化-脱合金以及硬/软模板方法制备了各种多孔纳米结构金,但成功合成具有可定制尺寸和孔隙率的mesoAu纳米球仍然是一个巨大的挑战。在这里,我们描述了一种新型的表面活性剂定向合成路线,以通过使用C 22 H 45 N +(CH 3)2 –C 3 H 6 –SH(Cl−)(C 22 N–SH)作为中孔导向剂。Ç 22 N-SH不仅可以自三价降低的Au( III)氯4 -单价金(我),但也形成聚合Ç 22 N-S-AU(我)的中间体通过共价键。这些C 22 N–S–Au( I)中间体有助于自组装成球形胶束并抑制Au前体的迁移,从而通过原位形成mesoAu纳米球的结晶成核和生长化学还原。合成策略可以进一步扩展以定制mesoAu纳米球的尺寸/孔隙率和表面光学性质。mesoAu纳米球在甲醇电氧化中表现出显着增强的质量/比活性和改善的稳定性,证明其性能远远优于无孔的Au纳米颗粒和先前报道的Au纳米催化剂。合成路线与其他长期建立的软模板方法截然不同,这为生长具有所需纳米结构和功能的金属纳米晶体提供了一条新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号