European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-05-28 , DOI: 10.1016/j.ejmech.2019.05.062
Rosa Purgatorio , Modesto de Candia , Marco Catto , Antonio Carrieri , Leonardo Pisani , Annalisa De Palma , Maddalena Toma , Olga A. Ivanova , Leonid G. Voskressensky , Cosimo D. Altomare
|
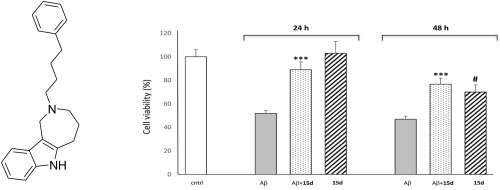
Due to the role of butyrylcholinesterase (BChE) in acetylcholine hydrolysis in the late stages of the Alzheimer's disease (AD), inhibitors of butyrylcholinesterase (BChE) have been recently envisaged, besides acetylcholinesterase (AChE) inhibitors, as candidates for treating mild-to-moderate AD. Herein, synthesis and AChE/BChE inhibition activity of some twenty derivatives of 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole (HHAI) is reported. Most of the newly synthesized HHAI derivatives achieved the inhibition of both ChE isoforms with IC50s in the micromolar range, with a structure-dependent selectivity toward BChE. Apparently, molecular volume and lipophilicity do increase selectivity toward BChE, and indeed the N2-(4-phenylbutyl) HHAI derivative 15d, which behaves as a mixed-type inhibitor, resulted the most potent (IC50 0.17 μM) and selective (>100-fold) inhibitor toward either horse serum and human BChE. Moreover, 15d inhibited in vitro self-induced aggregation of neurotoxic amyloid-β (Aβ) peptide and displayed neuroprotective effects in neuroblastoma SH-SY5Y cell line, significantly recovering (P < 0.001) cell viability when impaired by Aβ1-42 and hydrogen peroxide insults. Overall, this study highlighted HHAI as useful and versatile scaffold for developing new small molecules targeting some enzymes and biochemical pathways involved in the pathogenesis of AD.
中文翻译:

研究1,2,3,4,5,6-六氢阿斯匹诺[4,3- b ]吲哚作为丁酰胆碱酯酶选择性抑制剂的支架,对阿尔茨海默氏病具有额外的神经保护活性
由于阿尔茨海默氏病(AD)晚期丁酰胆碱酯酶(BChE)在乙酰胆碱水解中的作用,除乙酰胆碱酯酶(AChE)抑制剂外,最近还设想了丁酰胆碱酯酶(BChE)抑制剂可作为治疗轻度至中度肝炎的候选药物。适度的广告。在此,报道了1,2,3,4,5,6-六氢az庚啶[4,3- b ]吲哚(HHAI)的约二十种衍生物的合成和AChE / BChE抑制活性。大多数新合成的HHAI衍生物都可以在微摩尔范围内用IC 50抑制两种ChE异构体,并且对BChE具有结构依赖性的选择性。显然,分子体积和亲脂性确实增加了对BChE的选择性,实际上增加了N 2-(4-苯基丁基)HHAI衍生物的选择性。充当混合型抑制剂的15d对马血清和人BChE产生了最有效的(IC 50 0.17μM)和选择性的(> 100倍)抑制剂。此外,15d抑制神经毒性淀粉样β(Aβ)肽的体外自我诱导聚集,并在神经母细胞瘤SH-SY5Y细胞系中显示出神经保护作用, 当被Aβ1-42和过氧化氢损伤时可显着恢复(P <0.001)细胞活力。侮辱。总的来说,这项研究强调了HHAI是一种有用且用途广泛的支架,可用于开发针对AD发病机理中涉及的某些酶和生化途径的新小分子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号