Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2019-05-28 , DOI: 10.1016/j.jorganchem.2019.05.023 Vitor H. Menezes da Silva , Nelson H. Morgon , Carlos R.D. Correia , Ataualpa A.C. Braga
|
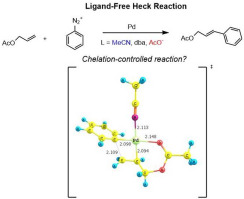
A density functional theory (DFT) study has been carried out with respect to the cationic and neutral mechanism of ligand-free Heck-Matsuda cross-coupling reaction involving allylic esters and arenediazonium salts. Full Heck-Matsuda (HM) catalytic cycles were explored in the presence of different possible combinations of ligands based on previous mass-spectrometry studies. DFT calculations were performed to examine the olefinic substrate influences on the regioselectivity of this ligand-free Heck reaction. A multi-step mechanism was revealed for the oxidative addition of palladium (0) center to carbon-nitrogen bond of arenediazonium cation. The cationic migratory insertion pathway is favored by a chelated coordination involving the palladium center and the allylic ester. In contrast, for neutral complexes, a bidentate coordination of acetate ligand is the favored pathway. Regarding the hydride elimination step, the resulting free energy profile is quite flat, revealing that the Heck product and the hydride complex are easily formed for both cationic and neutral cycles. The reductive elimination step assisted by acetate is a very exothermic process, going through small relative reaction barriers. These results constitute new insights to a better understanding of the selectivity and the mechanism of ligand-free Heck-Matsuda reactions.
中文翻译:

DFT透视涉及烯丙基酯和槟榔重氮盐的无配体Heck反应的选择性和机理
对于涉及烯丙基酯和槟榔二鎓盐的无配体的Heck-Matsuda交叉偶联反应的阳离子和中性机理,已经进行了密度泛函理论(DFT)研究。基于先前的质谱研究,在存在不同可能的配体组合的情况下,探索了完整的Heck-Matsuda(HM)催化循环。进行DFT计算以检查烯烃底物对这种无配体的Heck反应的区域选择性的影响。揭示了将钯(0)中心氧化加成到芳族重氮阳离子的碳-氮键上的多步机理。阳离子迁移插入途径受涉及钯中心和烯丙基酯的螯合配位的青睐。相反,对于中性配合物,乙酸盐配体的双齿配位是优选的途径。关于氢化物消除步骤,所得的自由能分布曲线相当平坦,表明对于阳离子和中性循环而言,很容易形成Heck产物和氢化物配合物。乙酸盐辅助的还原消除步骤是一个非常放热的过程,它会经历较小的相对反应障碍。这些结果构成了新的见解,可以更好地理解无配体的Heck-Matsuda反应的选择性和机理。经历较小的相对反应障碍。这些结果构成了新的见解,可以更好地理解无配体的Heck-Matsuda反应的选择性和机理。经历较小的相对反应障碍。这些结果构成了新的见解,可以更好地理解无配体的Heck-Matsuda反应的选择性和机理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号