Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2019-05-25 , DOI: 10.1016/j.cej.2019.05.165 Jonghun Lim 1 , Michael R Hoffmann 1
|
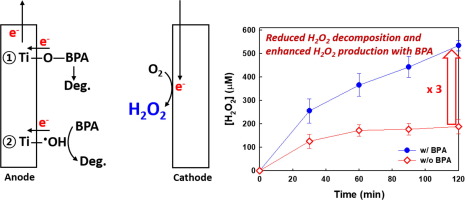
Hydrogen peroxide (H2O2) is electrochemically produced via oxygen (O2) reduction on a carbon cathode surface. In order to enhance the production of H2O2, anodic loss pathways, which significantly reduce the overall H2O2 production rate, should be inhibited. In this study, we investigate the effects of organic electron donors (i.e., typical chemical contaminants) on the anodic loss pathways of H2O2 in a single-cell electrochemical reactor that employs an anode composed of TiO2 over-coated on a mixed-metal oxide ohmic contact catalyst, Ir0.7Ta0.3O2, deposited on a Ti-metal that is coupled with a graphite rod cathode in a sodium sulfate (Na2SO4) electrolyte that is saturated with oxygen (O2). Organic electron donors are shown to enhance the electrochemical production of H2O2, while simultaneously undergoing oxidative degradation. The observed positive effect of organic electron donors on the electrochemical production of H2O2 is due in part to a preferential adsorption of organic substrates on the TiO2 outer layer of the anode. The sorption of the organic electron donors inhibits the formation of surficial titanium hydroperoxo species (Ti-OOH) on the anode surface. The organic sorbates also act as scavengers of surface-bound hydroxyl radical
Ti-OH. As a result, the decomposition of H2O2 on the anode surface is significantly reduced. The cathodic production rate of H2O2 at low pH is enhanced due to proton coupled electron transfer (PCET) to O2, while the anodic decomposition of H2O2 is inhibited due to electrostatic interactions between negatively-charged organic substrates and a positively-charged outer surface of the anode (TiO2 pHzpc = 5.8) at low pH.
中文翻译:

底物氧化增强了过氧化氢的电化学生产
过氧化氢 (H 2 O 2 ) 是通过在碳阴极表面上的氧 (O 2 ) 还原而电化学产生的。为了提高H 2 O 2 的产生,应抑制显着降低整体H 2 O 2产率的阳极损失途径。在这项研究中,我们研究了有机电子供体(即典型的化学污染物)对单电池电化学反应器中H 2 O 2阳极损失途径的影响,该反应器采用由 TiO 2组成的阳极覆盖在混合材料上。 -金属氧化物欧姆接触催化剂,Ir 0.7Ta 0.3 O 2 ,沉积在钛金属上,该钛金属与在氧饱和的硫酸钠 (Na 2 SO 4 ) 电解质中的石墨棒阴极耦合 (O 2 )。有机电子供体显示可增强 H 2 O 2的电化学产生,同时进行氧化降解。观察到的有机电子供体对电化学生产 H 2 O 2的积极影响部分是由于有机底物优先吸附在 TiO 2上阳极的外层。有机电子供体的吸附抑制了阳极表面表面氢过氧钛物质 (Ti-OOH) 的形成。有机山梨酸盐还充当表面结合的羟基自由基
Ti-OH 的清除剂。结果,阳极表面上H 2 O 2的分解显着减少。由于质子耦合电子转移 (PCET) 到 O 2 ,在低 pH 条件下 H 2 O 2的阴极产率得到提高,而由于带负电的有机底物和 A 之间的静电相互作用,抑制了 H 2 O 2的阳极分解。阳极外表面带正电(TiO2 pH zpc = 5.8) 在低 pH 条件下。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号