当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Chlorine-Atom-Controlled Terminal-Epoxide-Initiated Bicyclization Cascade Enables a Synthesis of the Potent Cytotoxins Haterumaimides J and K
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-05-26 , DOI: 10.1021/jacs.9b04702 Sharon E Michalak 1 , Sangkil Nam 2 , David M Kwon 2 , David A Horne 2 , Christopher D Vanderwal 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-05-26 , DOI: 10.1021/jacs.9b04702 Sharon E Michalak 1 , Sangkil Nam 2 , David M Kwon 2 , David A Horne 2 , Christopher D Vanderwal 1
Affiliation
|
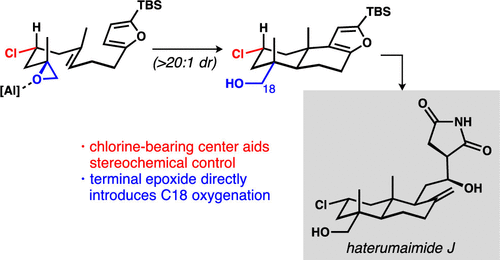
Haterumaimide J (hatJ) is reportedly the most cytotoxic member of the lissoclimide family of labdane diterpenoids. The unusual functional group arrangement of hatJ-C18 oxygenation and C2 chlorination-resisted our efforts at synthesis until we adopted an approach based on rarely studied terminal epoxide-based cation-π bicyclizations that is described herein. Using the C2-chlorine atom as a key stereocontrol element and a furan as a nucleophilic terminator, the key structural features of hatJ were rapidly constructed. The 18-step stereoselective synthesis features applications of chiral pool starting materials, and catalyst-, substrate-, and auxiliary-based stereocontrol. Access to hatJ and its acetylated congener hatK permitted their biological evaluation against aggressive human cancer cell lines.
中文翻译:

氯原子控制的末端环氧化物引发的双环化级联能够合成有效的细胞毒素 Haterumaimides J 和 K
据报道,Haterumaimide J (hatJ) 是拉丹烷二萜类 lissoclimide 家族中细胞毒性最强的成员。hatJ-C18 氧化和 C2 氯化的不寻常官能团排列阻碍了我们的合成努力,直到我们采用了一种基于本文所述的很少研究的基于末端环氧化物的阳离子-π 双环化的方法。使用 C2-氯原子作为关键立体控制元素和呋喃作为亲核终止剂,快速构建了 hatJ 的关键结构特征。18 步立体选择性合成的特点是应用手性池起始材料,以及基于催化剂、底物和助剂的立体控制。获得 hatJ 及其乙酰化同源物 hatK 允许他们对侵袭性人类癌细胞系进行生物学评估。
更新日期:2019-05-26
中文翻译:

氯原子控制的末端环氧化物引发的双环化级联能够合成有效的细胞毒素 Haterumaimides J 和 K
据报道,Haterumaimide J (hatJ) 是拉丹烷二萜类 lissoclimide 家族中细胞毒性最强的成员。hatJ-C18 氧化和 C2 氯化的不寻常官能团排列阻碍了我们的合成努力,直到我们采用了一种基于本文所述的很少研究的基于末端环氧化物的阳离子-π 双环化的方法。使用 C2-氯原子作为关键立体控制元素和呋喃作为亲核终止剂,快速构建了 hatJ 的关键结构特征。18 步立体选择性合成的特点是应用手性池起始材料,以及基于催化剂、底物和助剂的立体控制。获得 hatJ 及其乙酰化同源物 hatK 允许他们对侵袭性人类癌细胞系进行生物学评估。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号