当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Protonation of Enol Esters with Bifunctional Phosphonium/Thiourea Catalysts
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-24 00:00:00 , DOI: 10.1021/acs.orglett.9b01216 Eiji Yamamoto 1 , Kodai Wakafuji 1 , Yusuke Mori 1 , Gaku Teshima 1 , Yuki Hidani 1 , Makoto Tokunaga 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-24 00:00:00 , DOI: 10.1021/acs.orglett.9b01216 Eiji Yamamoto 1 , Kodai Wakafuji 1 , Yusuke Mori 1 , Gaku Teshima 1 , Yuki Hidani 1 , Makoto Tokunaga 1
Affiliation
|
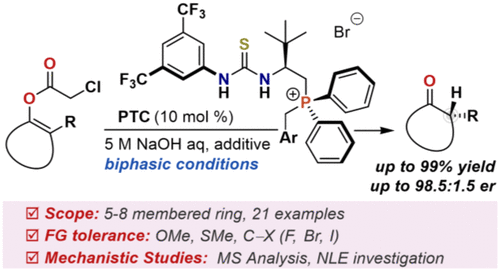
Bifunctional phosphonium/thioureas derived from tert-leucine behaved as highly selective catalysts for enantioselective protonation of enol esters, providing α-chiral ketones in yields of up to 99% with high enantioselectivities (up to 98.5:1.5 er). Control experiments clarified that a bulky tert-butyl group and phosphonium and thiourea moieties were necessary to achieve such high stereoselectivity. In addition, mechanistic investigations indicated the catalyst was converted to the corresponding betaine species, which served as a monomolecular catalyst.
中文翻译:

双功能Ph /硫脲催化剂对烯醇酯的对映选择性质子化
衍生自叔亮氨酸的双官能phospho /硫脲可作为烯醇酯对映选择性质子化的高选择性催化剂,以高对映选择性(高达98.5:1.5 er)提供α-手性酮,收率高达99%。对照实验表明,大的叔丁基基团以及phospho和硫脲部分对于实现如此高的立体选择性是必需的。此外,机理研究表明,该催化剂已转化为相应的甜菜碱,可作为单分子催化剂。
更新日期:2019-05-24
中文翻译:

双功能Ph /硫脲催化剂对烯醇酯的对映选择性质子化
衍生自叔亮氨酸的双官能phospho /硫脲可作为烯醇酯对映选择性质子化的高选择性催化剂,以高对映选择性(高达98.5:1.5 er)提供α-手性酮,收率高达99%。对照实验表明,大的叔丁基基团以及phospho和硫脲部分对于实现如此高的立体选择性是必需的。此外,机理研究表明,该催化剂已转化为相应的甜菜碱,可作为单分子催化剂。













































 京公网安备 11010802027423号
京公网安备 11010802027423号