当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BF3·OEt2-Promoted Propargyl Alcohol Rearrangement/[1,5]-Hydride Transfer/Cyclization Cascade Affording Tetrahydroquinolines
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-22 00:00:00 , DOI: 10.1021/acs.orglett.9b01153 Shuang Zhao 1 , Xiaoyang Wang 1 , Pengfei Wang 1 , Guangwei Wang 1 , Wentao Zhao 1 , Xiangyang Tang 1 , Minjie Guo 2
Organic Letters ( IF 4.9 ) Pub Date : 2019-05-22 00:00:00 , DOI: 10.1021/acs.orglett.9b01153 Shuang Zhao 1 , Xiaoyang Wang 1 , Pengfei Wang 1 , Guangwei Wang 1 , Wentao Zhao 1 , Xiangyang Tang 1 , Minjie Guo 2
Affiliation
|
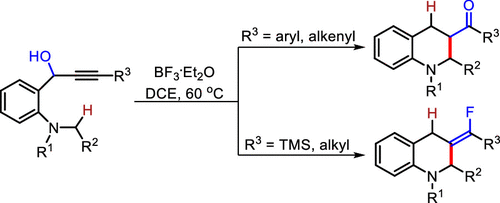
An efficient BF3·OEt2-mediated propargyl alcohol rearrangement/[1,5]-hydride transfer/cyclization cascade for the synthesis of tetrahydroquinoline derivatives has been described. The substituents adjacent to triple bonds play an important role in the formation of ketones (via [1,3]-hydroxyl shift) or alkenyl fluorides which are products of formal trans-carbofluorination of internal alkynes. This method provides a rapid access to diverse heterocycles in moderate to excellent yields.
中文翻译:

BF 3 ·OEt 2-促进的炔丙醇重排/ [1,5]-氢化物转移/环化级联四氢喹啉
已经描述了用于合成四氢喹啉衍生物的有效的BF 3 ·OEt 2介导的炔丙醇重排/ [1,5]-氢化物转移/环化级联反应。与三键相邻的取代基在酮(通过[1,3]-羟基转移)或链烯基氟化物的形成中起着重要作用,它们是内部炔烃的正式反式-碳氟化作用的产物。该方法可以以中等到极好的收率快速访问各种杂环。
更新日期:2019-05-22
中文翻译:

BF 3 ·OEt 2-促进的炔丙醇重排/ [1,5]-氢化物转移/环化级联四氢喹啉
已经描述了用于合成四氢喹啉衍生物的有效的BF 3 ·OEt 2介导的炔丙醇重排/ [1,5]-氢化物转移/环化级联反应。与三键相邻的取代基在酮(通过[1,3]-羟基转移)或链烯基氟化物的形成中起着重要作用,它们是内部炔烃的正式反式-碳氟化作用的产物。该方法可以以中等到极好的收率快速访问各种杂环。































 京公网安备 11010802027423号
京公网安备 11010802027423号